Translate this page into:
Review on mucormycosis – A gloom epoch

*Corresponding author: Saramma Mathew Fenn, Associate Professor, Department of Oral Medicine and Radiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India. docere_saramathew@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Raju FV, Fenn SM, Mohan KR, Kumar R. Review on mucormycosis – A gloom epoch. J Acad Dent Educ 2022;8:37-41.
Abstract
Mucormycosis recently known with the term as black fungus belongs to the Zygomycetes family. It is a filamentous non-septate fungus. Mucormycosis is not a novel disease, although it is becoming more well-known as a result of the extensive transmission of COVID-19-associated mucormycosis. COVID-19 is currently undergoing a catastrophic phase, which is being exacerbated by the devastating spread of mucormycosis. Mucormycosis identifies the target site by exploiting conditions such as immunocompromised health, steroid therapy, and diabetes that predispose patients to infection. Mucormycosis is more prevalent in India due to the relatively high percentage of diabetics in the population causing cutaneous mucormycosis, pulmonary mucormycosis, rhino-orbital cerebral mucormycosis, and gastrointestinal mucormycosis. It is a potentially fatal condition, and this review will provide an overview of the causative organism and its effects on human lives.
Keywords
Mucormycosis
Rhinocerebral mucormycosis
Diabetes
Hyperferritinemia
INTRODUCTION
Mucormycosis, a saprophytic aerobic fungus, has ushered in a dark age over the past 2 years. COVID-19 has caused a major crisis in the health-care system and has amplified the pressure on the system which led to the term as BLACK FUNGUS referred by the common people.[1] Mucormycosis is more associated with patients with comorbidities such as immunocompromised conditions, diabetics, and people on steroid medication, pulmonary disease, and so on. All of this can pave the path for the formation of other opportunistic illnesses from the same fungus kingdom.
Mucormycosis is not a new disease; it has been around for a long time, but it was a hidden entity. We cannot reason it as a newly developed condition; however, it is an uncommon angio-obtrusive disease.[2] The fungus itself survives by decomposing organic matter and then intensifying their growth. High glucose, low oxygen, acidic medium, high iron levels, and decreased phagocytic activity of white blood cells appear to be the optimal and ideal setting for Mucorales spore germination.[3] Mucorales fungi are the most frequent mold pathogen after Aspergillus.[4] The most common form of mucormycosis is rhinocerebral of maxillofacial region. Orofacial mycotic coinfections are reported with increasing frequency, occurring in conjunction with COVID-19, where the fungal invasion by the fungal spores entering through the nasal or oral mucosa spread to maxillary sinus with an involvement of maxillary alveolar bone tissues of the oral cavity and the orbital invasion with the later spread to the cavernous sinus by hematogenous dissemination.[5]
MICROBIOLOGY OF MUCORMYCOSIS
Mucormycetes are a type of mold that causes mucormycosis (Zygomycosis).[6,7] Mucoromycotina is the subphylum, and it is part of the Mucorales order. Mucormycosis is caused by 11 genus and 27 species of Mucorales. Rhizopus is the most common genus that causes mucormycosis. Rhizopus, Lichthemia, and Mucor cause three-quarter of mucormycosis cases.[8] Mucorales are commonly found in dust, soil, manure, and decaying food. The Castor bean (Ricinus communis) plant produces ricin, which has structural and functional similarities to the toxin.[9]
Rhizopus and Mucor species are known to cause rhino-orbital cerebral mucormycosis. These fungi are angioinvasive in nature that causes tissue necrosis and thrombosis as described in [Figure 1]. Fungal spread and cell damage increase as the interaction of receptors of endothelial cells with fungal spores increases by expressing spore coat protein homologs(CotH3), a member of mitogen activated protein causing fungal invasion. However the spread was earlier considered as when the Mucorales invade human umblical vein endothelial cells with the expression of CotH3 and interaction of glucose regulated protein (GRP78) and 78 Da host receptor thereby leading to angioinvasion.[9]
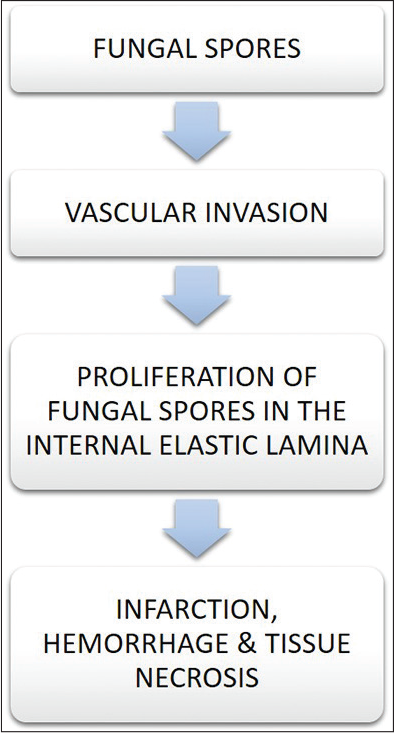
- Effect of fungal invasion.
PREDISPOSING FACTORS
Various studies are conducted globally and nationally to identify the predisposing factors of mucormycosis. Based on the prospective study done in Europe and Italy, it is seen that, among the mucormycosis patients, most of them have underlying conditions such as diabetes mellitus with or without diabetic ketoacidosis, hematological malignancies, trauma, and AIDS. Various other studies have revealed that factors such as transplants, other malignancies, and corticosteroids, iron overload, malnutrition, and immunocompromised conditions are known to favor infection.
PATHOPHYSIOLOGY
The invasion of mucormycosis involves several mechanisms and the following paragraphs will attempt to detail the pathophysiology.
Individuals have their own built-in immunity to combat spores and eradicate them from mucosal surfaces.[10,11] Mononuclear and polymorphonuclear phagocytes are clearly the host’s primary defense mechanism, acting against the organism through oxidative metabolites and cationic defensins.[12] Phagocytes destroy foreign substances by oxidative and non-oxidative mechanisms. However, certain situations, such as hyperglycemia and acidosis, impair phagocytes’ ability to operate against foreign materials. Neutrophil and lymphocytic abnormalities are two other risk factors for mucormycosis. Individuals with diabetes who have ketoacidosis have a higher risk of getting mucormycosis than patients who do not have diabetic ketoacidosis.[13]
The metabolic requirements for the growth of fungi depends on host iron exclusively. Increased serum iron in the host will increase the mucormycosis susceptibility. Altered iron hemostasis is seen in patients taking iron chelator deferoxamine and is more prone to mucormycosis. For the growth and benefit of fungus, it uses chelated iron as a siderophore. An increased level of serum iron is seen in patients with diabetic ketoacidosis. The ability of transferrin to chelate iron is compromised by beta-hydroxybutyrate (ketoacid). The ability of transferrin to chelate iron is compromised by beta-hydroxyburate in diabetic ketoacidosis condition where the availability of free iron is increased by preventing the iron sequestration by transferrin and ferritin. Viral infection causes endocytosis through hemoglobin, which results in hemoglobin dysfunction, hemolysis, and decreased oxygen transport [Figure 2].
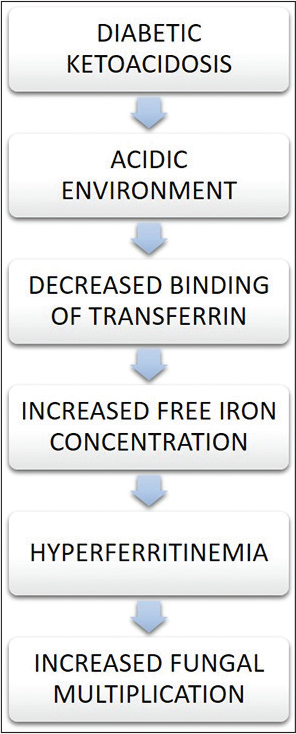
- Relation between diabetic ketoacidosis and iron.
Tissue necrosis and thrombosis of vessels are the consequences of angioinvasion by the fungus which adheres to the extracellular matrix protein laminin after entering the skin or mucosa. With the help of a specific receptor GRP78, fungus interacts with endothelial cells and causes fungal endocytosis.[14]
Corticosteroids,which are potent immunosuppressants,can act as a predisposing factor for COVID19-associated mucormycosis.[15] Studies have shown at the time of COVID-19 treatment, diabetes patients who were treated with corticosteroids showed an increased incidence of mucormycosis. Min et al. evaluated a retrospective study in a cohort of 500 patients regarding the adverse effects of short-term high-dose steroid therapy. In one-third of the patients, limited effects are noted. However, in patients with long-term steroid administration, irrespective of the dose, many opportunistic infections develop.
DISCUSSION
COVID-19-associated mucormycosis
Mucormycosis is one of the numerous opportunistic infections seen during COVID-19. Several studies have shown that uncontrolled diabetes mellitus and diabetic ketoacidosis are the major risk factors to develop this disease. Increased free iron levels in the serum as a result of hyperglycaemia and a hyperacidic state can predispose to mucormycosis. COVID-19 creates some favorable conditions for mucormycosis. Hyperglycemia, hyperferritinemia, hypoxia, attenuated phagocytic activity, etc., are the favorable conditions produced by COVID-19 and cause immunosuppression.[16] Artis et al. reported that the susceptibility of endothelial cells to Rhizopus is increased by high glucose and iron levels.
The immune system of the individual is suppressed by the corticosteroids that were given during the COVID-19 treatment.[17] Mucormycosis together with COVID-19 is actually a deadly companion. The nose, sinuses, orbit, CNS, lungs, mediastinum, GIT, jaw bones, skin, heart, kidney, joints, and abdominal portion are the target sites of mucormycosis. In both diabetic and non-diabetic patients, the species found was Rhizopus. Rhizopus thus plays an important role in the mucormycosis caused by COVID-19. Patients are more prone to secondary infections such as mucormycosis due to the series of events that are caused by COVID-19. In severe cases of COVID-19, there is a decrease in CD4+ and CD8+ groups, which predisposes to mucormycosis as a result of immunosuppression. The mortality rate of 30.73% in patients with COVID-19 and mucormycosis was reported by SeyedAlinaghi, S et al.[18] AIDS, organ transplantation, malignancies, neutropenia, iron overload, malnutrition, etc., can also predispose to mucormycosis. Through the enhanced production of stress hormones, insulin resistance increases in severe COVID-19. Steroids play an unavoidable role in COVID-19-associated mucormycosis.
Diabetes and mucormycosis
From various studies conducted on different geographical areas, it is seen that different geographical areas have considerably different risk factors. Diabetes mellitus was a risk factor in India, Iran, and Mexico, as well as hematological malignancy in Europe. Stemler et al. conducted studies in countries in the Middle East and North Africa. This study reveals that diabetes mellitus was one of the leading underlying conditions for mucormycosis. As the number of diabetes mellitus cases increases, it is expected that there will be an increase in mucormycosis cases. In India, the majority of the mucormycosis cases were reported and also in patients with uncontrolled diabetes mellitus as the major risk factor in these cases. A multicenter study was conducted on mucormycosis in India recently. This study reveals that 57% of patients have uncontrolled diabetes and an additional 18% have diabetic ketoacidosis.[19] The morbidity and mortality of COVID-19 increase when diabetes mellitus acts as the major risk factor. In his review, Jeong et al. revealed that in 40% of cases, the underlying condition is diabetes mellitus and diabetic ketoacidosis in 20% of cases.
In patients with uncontrolled diabetes mellitus, the most common occurrence is rhino-orbital cerebral mucormycosis, because high blood sugar is a favorable environment for growth and flourishment of Rhizopus.[20] The acidic pH and increased free iron concentration will be an important factor for rhino-orbital cerebral mucormycosis. Diabetic ketoacidosis leads to uncontrolled hyperglycemia, which causes a drastic change in the immune response of the individual. In addition to that, the enzyme ketone reductase from Mucorales will increase the glucose level and diabetic ketoacidosis. According to a review by John et al., diabetes mellitus was an underlying condition in 93% of cases of mucormycosis. In some patients, a euglycemic state was also found. In all the aspects of COVID-19 infection, diabetes stands as a separate or independent risk factor for mucormycosis specifically in rhino-orbital cerebral mucormycosis.
Overall mortality is high for rhino-orbital cerebral mucormycosis. It is an angioinvasive fungal infection that affects brain and paranasal sinuses. Immunocompromised patients are at high risk for developing rhino-orbital cerebral mucormycosis,[21] as depicted in [Figures 1 and 3].
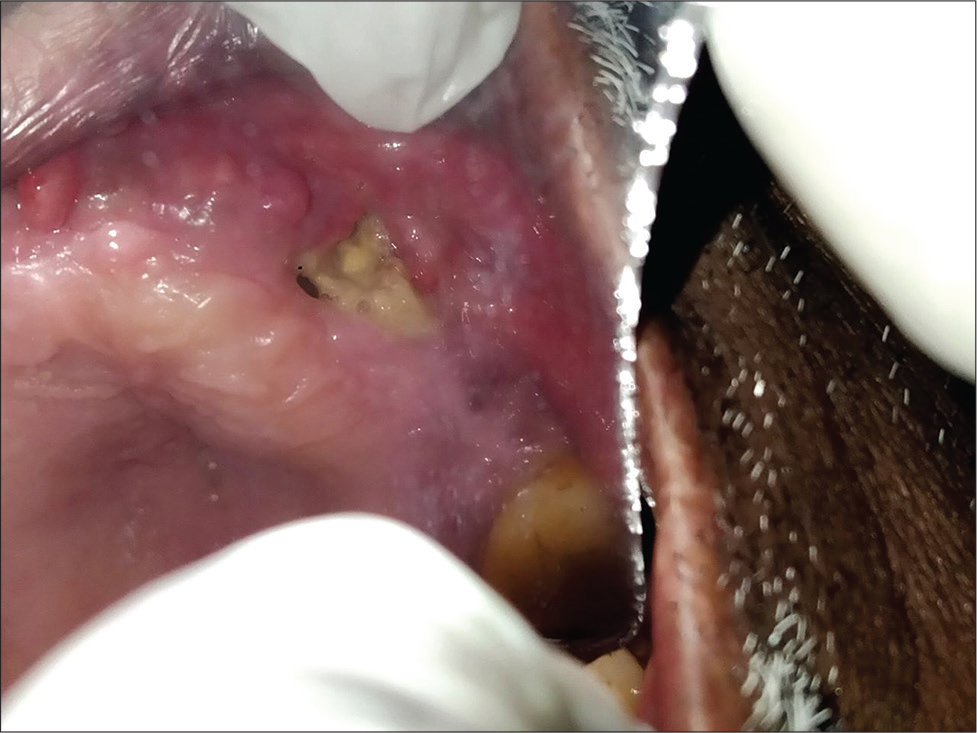
- Mucormycosis invasion in hard palate.
Earlier diagnosis is needed for this type of mucor invasion. Clinical manifestations, radiological findings and Histological analysis aid in diagnosis. The most important microscopic feature is broad-based ribbon-like non-septate hyphae with irregular right-angled branching, which acts as a key diagnostic feature.[22] To find out the extent of the disease, computed tomography (CT) imaging should be done. Internal carotid artery and cavernous sinus thrombosis, bony erosions, and ethmoid or sphenoid sinusitis with orbital or intracranial extensions are the associated pathological findings.[23] Glycemic control should be strictly followed for the management of rhinocerebral mucormycosis.
Investigations
Clinical signs, histological findings, and radiological results aid in diagnosis. The most frequent locations for specimen collection are the paranasal sinus and nasal cavity. Broad aseptate hyphae are visible histologically.[24] The visualization of fungal hyphae is improved with the use of flurorescent brightners,potassium hydroxide and Lactophenol cotton blue stain. Also for the histological examination hematoxylin and eosin, Periodic acid schiff, Grocott-Gomori methanamine silver stains are used. In computed tomography the classical finding of mucor invasion is seens as reverse halo sign and in immunodeficient mucormycosis affected patients the CT will show nodules with a halo of ground-glass opacity around them. In addition, a number of molecular techniques are becoming available for fungal diagnosis. Several PCR-based methods have been developed for the detection of fungal spores.[25] Burnha-Maurish et al. have stated that in the sandwich ELISA technique, a monoclonal antibody is found to be highly reactive with purified glucomannan of species mucor. Afterward, lateral flow immunoassays were developed, which are more convenient than ELISA for the detection of fungal spores.
Management
The first important matter for the management of mucormycosis is the removal of predisposing factors. Metabolic abnormalities should be corrected as earlier as possible. Intravenous antifungal drugs are given. Amphotericin B at a dosage of 5–10 mg/kg/day is the first-line treatment for mucormycosis. Posaconazole intravenously is given as a second line of treatment. Isavuconazole is the other active antifungal drug which can be administered with surgical drainage and debridement to be done.
Limitations of the review
However, there are a few limitations to this review wherein the entire aspect of mucormycosis is not covered related to studies conducted and epidemiology of mucormycosis.
CONCLUSION
It is evident that COVID-19 enhances the risk and burden of COVID-19-associated mucormycosis, as well increasing the mortality rate in individuals with comorbidities. The most significant risk factors for mucormycosis include high blood sugar levels, high levels of serum free iron, low oxygen levels, and inadequate immunity. Thus, patient’s life can be preserved by early detection, antifungal treatment, and surgical management.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Dental and oral manifestations of COVID-19 related Mucormycosis: Diagnoses, management strategies and outcomes. J Fungi (Basel). 2021;8:44.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis: A new threat to Coronavirus disease 2019 with special emphasis on India. Clin Epidemiol Glob Health. 2022;15:101013.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15:102146.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and diagnosis of Mucormycosis: An update. J Fungi (Basel). 2020;6:265.
- [CrossRef] [PubMed] [Google Scholar]
- Orofacial mycoses in Coronavirus disease-2019 (COVID-19): A systematic review. Int Dent J. 2022;72:607-20.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis: An opportunistic pathogen during COVID-19. Environ Res. 2021;201:111643.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis and COVID-19 pandemic: Clinical and diagnostic approach. J Infect Public Health. 2022;15:466-79.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19-associated Mucormycosis: Evidence-based critical review of an emerging infection burden during the pandemic's second wave in India. PLoS Negl Trop Dis. 2021;15:e0009921.
- [CrossRef] [PubMed] [Google Scholar]
- Mucoricin is a ricin-like toxin that is critical for the pathogenesis of Mucormycosis. Nat Microbiol. 2021;6:313-26.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and pathophysiology of COVID-19-associated Mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186:739-54.
- [CrossRef] [PubMed] [Google Scholar]
- Hyperferritinemia and the extent of Mucormycosis in COVID-19 patients. Cureus. 2021;13:e20569.
- [CrossRef] [PubMed] [Google Scholar]
- Novel perspectives on Mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556-69.
- [CrossRef] [PubMed] [Google Scholar]
- Current understanding in the pathophysiology of SARS-CoV-2-associated rhino-orbito-cerebral Mucormycosis: A comprehensive review. J Maxillofac Oral Surg. 2021;20:373-80.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses. 2021;64:1253-60.
- [CrossRef] [PubMed] [Google Scholar]
- COVID 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab Syndr. 2021;15:102196.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral Mucormycosis in 2826 patients in India-collaborative OPAI-IJO study on Mucormycosis in COVID-19 (COSMIC), report 1. Indian J Ophthalmol. 2021;69:1670-92.
- [CrossRef] [PubMed] [Google Scholar]
- Black fungus immunosuppressive epidemic with Covid-19 associated Mucormycosis (Zygomycosis): A clinical and diagnostic perspective from India. Immunogenetics. 2022;74:197-206.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis infection in patients with COVID-19: A systematic review. Health Sci Rep. 2022;5:e529.
- [CrossRef] [Google Scholar]
- When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for Mucormycosis. J Fungi (Basel). 2021;7:298.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 triggering Mucormycosis in a susceptible patient: A new phenomenon in the developing world? BMJ Case Rep. 2021;14:e241663.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinocerebral Mucormycosis: A ten-year single centre case series. Cureus. 2020;12:e11776.
- [CrossRef] [Google Scholar]
- Challenges in the diagnosis and treatment of Mucormycosis. Med Mycol. 2018;56:93-101.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinocerebral Mucormycosis in COVID-19 patient with diabetes a deadly trio: Case series from the North-Western part of India. Indian J Med Microbiol. 2021;39:380-3.
- [CrossRef] [PubMed] [Google Scholar]
- Susceptibility of severe COVID-19 patients to rhino-orbital Mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65:515-25.
- [CrossRef] [PubMed] [Google Scholar]
- Association of COVID-19 with rhino-cerebral Mucormycosis: An observational study. J Maxillofac Oral Surg. 2022;21:990-4.
- [CrossRef] [PubMed] [Google Scholar]







