Translate this page into:
Prescription patterns of antibiotics and awareness of antibiotic resistance among dental house surgeons, postgraduate students, and dental professionals – A cross-sectional descriptive survey

*Corresponding author: Dr. Kiranmayi Govula, Professor, Department of Conservative Dentistry and Endodontics, Narayana Dental College and Hospital, Nellore, Andhra Pradesh, India. govulakiranmayi24@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Suchitha P, Pavan Kumar Y, Govula K, Modem V, Sannapureddy S, Maddineni MK. Prescription patterns of antibiotics and awareness of antibiotic resistance among dental house surgeons, postgraduate students, and dental professionals – A cross-sectional descriptive survey. J Academy Dent Educ. 2025;11:47-56. doi: 10.25259/JADE_48_2024
Abstract
Objectives
Exposing a patient to unnecessary antibiotics increases the risk that antibiotics will fail for that patient when required, thereby increasing the risk of antibiotic-resistant bacteria spreading to the patient’s family, friends, and other contacts. The study aimed to investigate the antibiotic prescribing habits of dental professionals, house surgeons, and postgraduates.
Material and Methods
The questionnaire consisted of 20 multiple-choice and two open-ended questions, organized into three main sections, which were explicitly investigated.
Results
Among the participants, 33.2% were males, and 66.8% were females. The work experience of practitioners ranged from 2-10 years. Among the volunteers, 60.4% were academicians, 24% had a private practice, 10% were employed as private employees, and 5.6% were employed as government employees. 37.2% were house surgeons, 17.2% were general dentists, 23.2% were postgraduates, and 22.4% had specialty practice.
Conclusion
The consultation of guidelines for antibiotic prescriptions varies, emphasizing the need for a more consistent approach within the practitioner community. Various sources, including textbooks (30.4%) and pharmaceutical guidelines (24%), contribute to their knowledge of antimicrobial resistance.
Keywords
Antibiotics
Awareness
Dentists
Emergencies
Periapical diseases
Pulpal diseases
Resistance
INTRODUCTION
Antimicrobial resistance (AMR) is not a local concern but a pressing global issue. The overuse and misuse of antibiotics lead to the development of resistance by microorganisms, making infections caused by these organisms challenging to treat with common antibiotics. The resistant bacteria can spread quickly between individuals and across countries, making antibiotic resistance a global issue. The condition became risky because of increases in the rate of hospitalization, leading to higher medical costs and increasing mortality rates. Understanding how antibiotics are prescribed and the level of awareness among dental professionals is crucial in addressing this global issue by promoting responsible antibiotic use.
During the pandemic, the spread of infections has become more widespread, and many infections did not even respond to the most prescribed antibiotics. According to the research, antibiotics taken by any patient can persist for up to 12 months in their microbiome. The bacteria may resist the causative drug and other drugs when a patient is exposed unnecessarily to antibiotics. Mainly, antibiotics were prescribed for a patient on demand or to be on the safe side; there is a risk of failure of antibiotics, especially when in need. The risk of antibacterial resistance slowly spreads to the patient’s family, friends, and contacts. Before prescribing antibiotics, doctors must be aware of the chances of developing resistance in that patient and thus can reduce the spread into society.
Antibiotics, when used more frequently, can lead to their resistance. Dentists constitute 10% of medical personnel across the globe who prescribe antibiotics.[1] Dentists prescribe antibiotics for almost every clinical scenario associated with pain.[2] 80% of antibiotics prescribed for treating acute dental conditions were found unnecessary according to a UK-based study,[3] and 80% of antibiotic use for prophylaxis was inappropriate according to research in the US.[4] After assessing the studies and surveys on the usage of antibiotics, it is confirmed that the dental profession is also responsible for the increase in antibiotic resistance and thereby held responsible for putting their efforts into preventing the spread.
The present survey is a crucial tool in understanding how antibiotics are prescribed by dental house surgeons, postgraduates, and general and specialty practitioners. This study is paramount as it can help identify trends and practices in antibiotic use, which can contribute to the global efforts to tackle antibiotic resistance. By collecting data on antibiotic prescription patterns and awareness, the survey can find the trends in the overuse and misuse of antibiotics. This helps in creating awareness of antibiotic resistance and decreases the load of antibiotic use. Therefore, study aimed to investigate the antibiotic prescribing habits of dental professionals, house surgeons, and postgraduates, aiming to promote responsible antibiotic use in dental practice.
The objectives were as follows:
To assess the attitudes toward prescribing antibiotics and, at the same time, AMR awareness
To know the levels of awareness and knowledge of antibiotic resistance
To evaluate areas of defect in knowledge and practices about antibiotic use
To assess the patterns in indicating the antibiotics and their prophylactic use for systemic conditions
To investigate the knowledge of misusing antibiotics
To evaluate adherence to guidelines
To identify educational needs.
MATERIAL AND METHODS
Study design
The present study aims to conduct an online and offline questionnaire survey of dentists in the Nellore Urban area, a region known for its diverse dental practices and patient demographics, from October 15 to November 15, 2023. The Nellore Urban area was chosen for its varied dental practices and patient demographics, which can provide a comprehensive understanding of antibiotic prescribing habits in different dental settings and patient populations.
Study participants
The participants were approached electronically and manually, and 250 participants were selected. The sample size is calculated using the formula.[5]
Sample Size Formula = [z2 * p(1-p)]/e2/1 + [z2 * p(1-p)]/e2 * N] Where,
N is the population size
z is the z-score
e is the margin of error
p is the standard deviation
Participation was voluntary and did not involve compensation. No personal health information or details were collected, participants’ data were protected, and no inhumane questions or investigations were involved.
Data collection
The questionnaire, developed after a literature review by dental surgeons from different dental specialties, consisted of 20 multiple-choice questions and two open questions, which were sorted into three main sections, specifically investigating:
Participants’ demographic features (their age and gender) and dental practice characteristics concerning their working experience (in years), dental setting (whether they worked in private practice alone, academician, or whether they were a public employee or a private employee), and their primary specialty for postgraduate students, and practitioners with M.D.S. degree (of oral surgery, endodontics, orthodontics, pediatric dentistry, periodontology, prosthetics/implantology, and others).
The following questions evaluated the antibiotic prescription habits of dentists:
How often do you prescribe systemic antibiotics?
Antibiotic therapy is prescribed mainly to treat bacterial infections. The duration of antibiotic therapy depends upon the type and severity of the infection to be treated and the age of the patient. Antibiotic prophylaxis can even be given before diagnosis, as in empiric therapy. It should be aimed at the causative organisms. If this is not possible, a combination of penicillin with aminoglycoside and metronidazole would be the best option. The most common side effects noticed after antibiotic use include nausea, diarrhea, and even stomach upset.
Dentists’ attitudes toward antibiotics and AMR awareness, whether they are aware of antibiotic resistance and how the organisms spread, how the infection caused by the microorganisms can be treated, dentists consult the guidelines for prescribing antibiotics, prescribing behavior regarding antibiotics and its relation to the development of antibiotic resistance, the main reason why antibiotics are prescribed without indication, and dentists’ primary source of reference to access information on the topic of antibiotic resistance and antibiotic therapy. After collecting data, an appropriate statistical analysis was performed.
RESULTS
A student t-test was performed after data were retrieved from the responses obtained through Google Forms. Demographic results showed that 33.2% are males, and 66.8% of females [Figure 1] and most of the participants age was below 30 years [Figure 2] responded that the work experience of practitioners ranged from <2 years to 72.8%. 2–10 years of [Figure 3] work experience is 20.8%. 60.4% are academicians, and 24% have private practice [Figure 3]. 10.0% are working as private employees, and 5.6% are working as public employees. 37.2% were house surgeons, 17.2% were general dentists, 23.2% were postgraduates, and 22.4% had specialty practice [Figure 4]. The details are given in detail in Table 1.
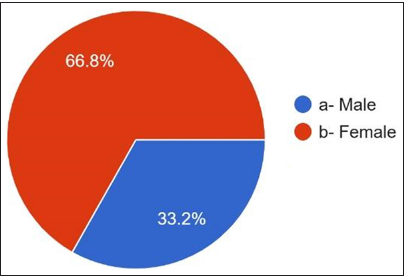
- Gender distribution in the study.
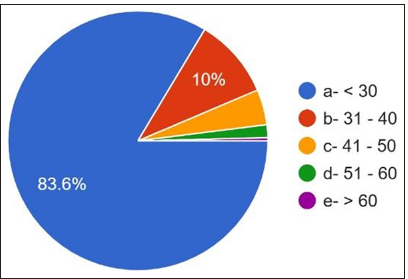
- Range of age of the participants.
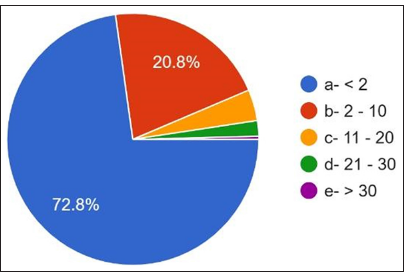
- Working experience of Participant.
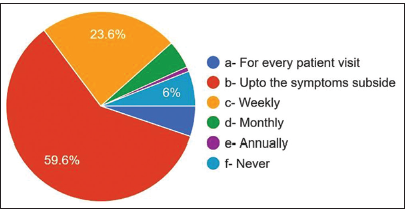
- Frequency of prescribing antibiotics.
| Demographics | Frequency | Percent |
|---|---|---|
| Gender | ||
| Male | 83 | 33.2 |
| Female | 167 | 66.8 |
| Age | ||
| < 2 | 182 | 72.8 |
| 2 – 10 | 52 | 20.8 |
| 11 – 20 | 10 | 4.0 |
| 21 – 30 | 5 | 2.0 |
| > 30 | 1 | 0.4 |
| Practice | ||
| Private practice | 60 | 24.0 |
| Academician | 151 | 60.4 |
| Public employee | 14 | 5.6 |
| Private employee | 25 | 10.0 |
| Specialization | 43 | 17.2 |
| General dentist | ||
| House surgeon | 93 | 37.2 |
| Postgraduate | 58 | 23.2 |
| M.D.S. | 56 | 22.4 |
M.D.S: Master of Dental Surgery
The overall responses were explained in detail in Table 2. 59.6% answered that they prescribed antibiotics up to symptoms subsided, 23.6% prescribed antibiotics weekly, 6% of dentists never prescribed antibiotics, 5.2% prescribed antibiotics for every patient visit, 4.8% prescribed antibiotics every month, and 0.8% of dentists prescribed antibiotics annually [Figure 5]. For the question of the most common cause of prescribing antibiotics, 58% answered periapical abscess, 16% answered pulpitis, only 10.8% answered cellulitis, and 10% answered periodontal abscess. 3.2% answered pericoronitis and trauma 2%.
| Questions | Frequency | Percentage |
|---|---|---|
| How often do you prescribe systemic antibiotic | ||
| a. For every patient visit | 13 | 5.2 |
| b. Upto the symptoms | 149 | 59.6 |
| c. Weekly | 59 | 23.6 |
| d. Monthly | 12 | 4.8 |
| e. Annually | 2 | 0.8 |
| f. Never | 15 | 6.0 |
| The most frequent cause of prescribing antibiotics | ||
| a. Pulpitis | 40 | 16.0 |
| b. Periapical abscess | 145 | 58.0 |
| c. Periodontal abscess | 25 | 10.0 |
| d. Cellulitis | 27 | 10.8 |
| e. Pericoronitis | 8 | 3.2 |
| f. Trauma | 5 | 2.0 |
| The most common therapy that requires antibiotics | ||
| a. Oral prophylaxis | 5 | 2.0 |
| b. Root canal therapy | 31 | 12.4 |
| c. Dental extraction | 183 | 73.2 |
| d. Root planning | 2 | 0.8 |
| e. Periodontal surgery | 20 | 8.0 |
| f. Implant surgery | 9 | 3.6 |
| Duration of antibiotic therapy ( days ) | ||
| a. 3 | 94 | 37.6 |
| b. 5 | 121 | 48.4 |
| c. 7 | 17 | 6.8 |
| d. > 7 | 4 | 1.6 |
| e. Till the symptoms subside | 14 | 5.6 |
| Most frequent antibiotics that you prescribe | ||
| a. Amoxicillin | 107 | 42.8 |
| b. Amoxicillin+clavulanic acid | 142 | 56.8 |
| c. Metronidazole | 1 | 0.4 |
| Antibiotics that you prescribe for penicillin allergy | ||
| a. Macrolides | 33 | 13.2 |
| b. Lincosamides | 1 | 0.4 |
| c. Fluoroquinolones | 5 | 2.0 |
| d. Cephalosporins | 90 | 36.0 |
| e. Metronidazole | 15 | 6.0 |
| f. Vancomycin | 3 | 1.2 |
| g. Clindamycin | 64 | 25.6 |
| h. Tetracycline | 29 | 11.6 |
| i. Don't know | 10 | 4.0 |
| The most common side effect you notice after antibiotic use | ||
| a. Gastritis | 127 | 50.8 |
| b. Nausea | 59 | 23.6 |
| c. Vomiting | 9 | 3.6 |
| d. Diarrhoea | 41 | 16.4 |
| e. Allergy | 14 | 5.6 |
| Dentists know what antibiotic resistance is | ||
| a. Yes | 242 | 96.8 |
| b. No | 8 | 3.2 |
| Antibioticresistant organisms transfer from person to person and animal to person and vice versa | ||
| a. Yes | 116 | 46.4 |
| b. No | 134 | 53.6 |
| Diseases caused by these antibioticresistant microorganisms are | ||
| a. Easily treatable | 44 | 17.6 |
| b. Difficult to treat | 206 | 82.4 |
| Do prescribing behavior antibiotics and the development of antibiotic resistancerelated | ||
| a. Yes | 157 | 62.8 |
| b. No | 23 | 9.2 |
| c. Don't know | 70 | 28.0 |
| How often do dentists consult guidelines for prescribing antibiotics | ||
| a. Always | 82 | 32.8 |
| b. Often | 87 | 34.8 |
| c. Occasionally | 73 | 29.2 |
| d. Never | 8 | 3.2 |
| The primary source consulted to obtain information on antimicrobial resistance and antibiotic administration | ||
| a. Textbooks | 76 | 30.4 |
| b. Forum on the internet | 31 | 12.4 |
| c. Scientific magazines | 15 | 6.0 |
| d. Consultation of guidelines | 49 | 19.6 |
| e. Direct communication with referring colleagues | 19 | 7.6 |
| f. Scientific information from the pharmaceutical guideline | 60 | 24.0 |
| The main reason why antibiotics are prescribed without indications | ||
| a. Noncompliant patients | 24 | 9.6 |
| b. Uncertainty of clinical condition | 38 | 15.2 |
| c. To avoid occurrence of flare up and emergency dental visit | 105 | 42.0 |
| d. To reduce the patient's discomfort | 83 | 33.2 |
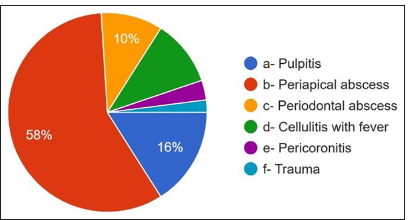
- Most frequent cause for antibiotic prescription.
The third question is the most common therapy requiring antibiotic therapy, and 73.2% answered dental extraction. 12.4% answered root canal therapy [Figure 6]. The duration of antibiotics showed that 48.4% were prescribed antibiotics for 5 days, 37.6% were prescribed for 3 days, and 6.8% were prescribed for 7 days. 1.6% were prescribed antibiotics >7 days, 5.6% answered up to symptoms subsiding, 56.8% of participants [Figure 7] were prescribed amoxicillin and clavulanic acid, and 42.8% were prescribed amoxicillin metronidazole, which is prescribed by only 0.4% [Figure 8].
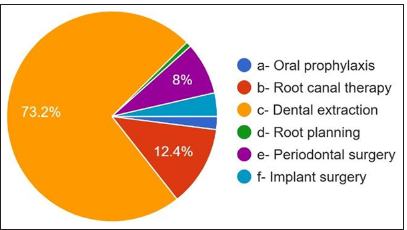
- Most suitable clinical situation for antibiotic prescription.
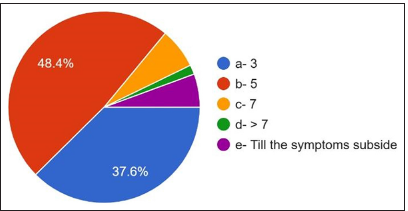
- Duration of antibiotic therapy.
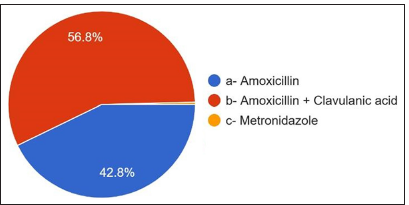
- Most common antibiotics prescribed.
Regarding the antibiotics prescribed for penicillin allergy, 36% answered cephalosporins, 25.6% answered clindamycin, and only 13.2% responded to macrolides [Figure 9]. They have noticed that the most common side effects [Figures 10 and 11] are gastritis 50.8%, nausea 23.6%, diarrhea 16.4%, allergy 5.6%, and vomiting 3.6%. 96.8% of dentists answered about antibiotic resistance, and 3.2% answered no [Figure 12]. For the question Antibiotic-resistant organisms transfer from person to person and animals to person and vice versa, 53.6% answered no, and 46.4% answered yes [Figure 13]. For diseases caused by these antibiotic-resistant microorganisms, 82.4% answered that they are challenging to treat. 17.6% answered that infections caused by antibiotic-resistant organisms are easily treatable [Figure 14].
- Percentage of Antibiotics prescription in various diseases.

- Antibiotics that prescribed in Penicillin allergy.
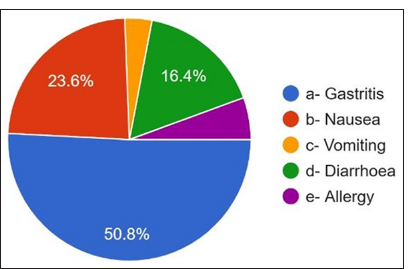
- Most common side effect after antibiotic use.
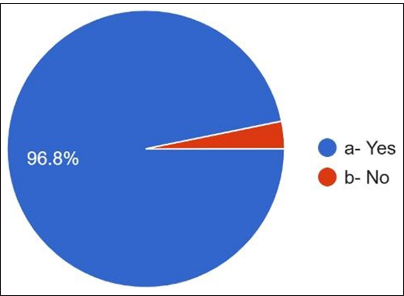
- Whether aware about antibiotic resistance.
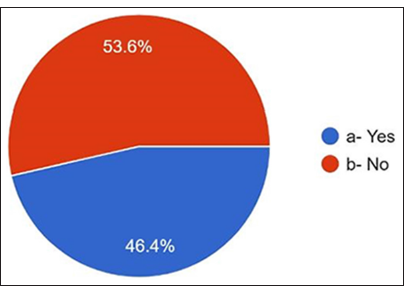
- Transfer of antibiotic reistant organisms.
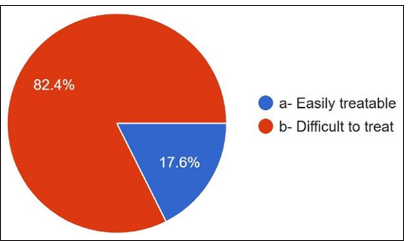
- Ability of treating the diseases caused by antibiotic resistant microorganisms.
Regarding the prescribing behavior of antibiotics and the development of antibiotic resistance, 62.8% answered yes, and 28% answered that they did not know [Figure 15]. 9.2% answered no. How often do dentists consult guidelines for prescribing antibiotics? 32.8% always answered, 34.8% usually answered, 29.2% responded occasionally, and 3.2% answered never [Figure 16].
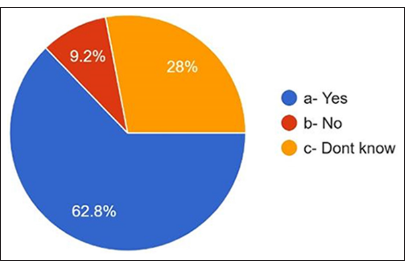
- Relation between prescribing antibiotics and antibiotic resistance.
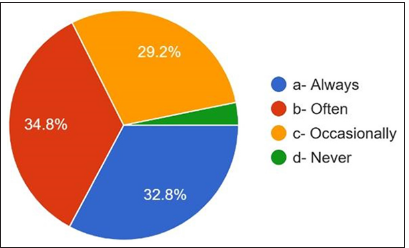
- Referring guidelines for antibiotic prescription.
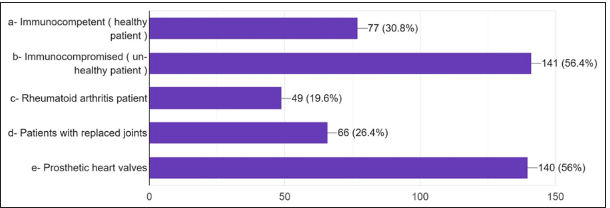
The primary source consulted to obtain information on AMR and antibiotic administration: 30.4% answered textbooks, 24% answered scientific information from pharmaceutical guidelines, 19.4% from consulting guidelines, 6% scientific magazines, and 10% from the internet [Figure 17]. For the main reason for prescribing antibiotics without indications, 42% answered to avoid complications [Figure 18]. 33.2% answered to prevent patient discomfort. For the question where the antibiotic prophylaxis was given, 30.8% answered immunocompetent healthy persons, 48.8% answered immunocompromised patients, 22% answered patients with rheumatoid arthritis, 26.8% responded to patients with replaced joints, patients with prosthetic heart valves in 55.56% [Figure 19].
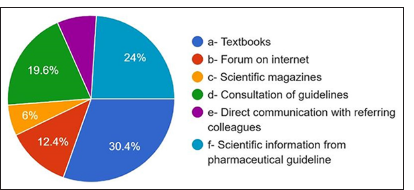
- Main source of information.
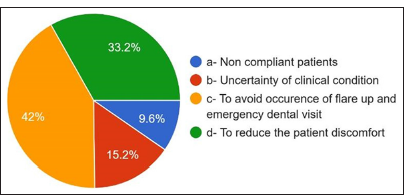
- Main reason for prescribing antibiotics without any indication.
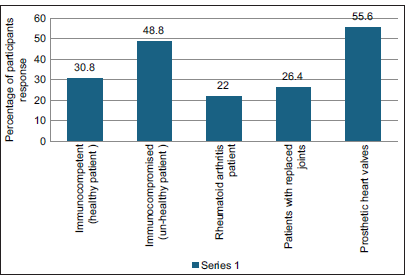
- Antibiotic prophylaxis is given in ( more than one option can be selected).
DISCUSSION
A study by Khalil et al. uncovered a pressing issue: a single dose of amoxicillin can significantly increase resistant strains and disrupt normal oral microflora within a day. This rapid disturbance, reducing normal flora and causing a surge in resistant bacteria, underscores the urgent need for action in this area.[6]
Many infections that several drug classes could successfully treat have acquired resistance. After exposure to antimicrobials, the microorganisms are subjected to selection pressure, where the resistant organisms are increased.[6] They acquire fitness by having and exhibiting the resistance genes, which they tend to share with the rest of the microorganisms’ poor infection control, environmental contamination, and geographical movement of infected humans and animals. There are reservoirs of resistance, including within humans and the local environments of hospitals and the community, as well as in animals and the farms and aquaculture environments, but also in water, soil, wildlife, and many other ecological niches due sewage waste pollution, pharma industrial waste, and manure run of from forms.[7]
AMR is an ecological problem characterized by complex interactions between the microbial population, which is diverse, and the environment.[8]
To help reduce antibiotic resistance, the World Health Organization (WHO) has introduced three classifications of antibiotics: the AWARE classification access, watch, and reserve.[9] The access group includes antibiotics that offer the best therapeutic value while minimizing the potential for resistance.[9] The watch group includes antibiotics that are more prone to selection for resistance. Antibiotics in the watch group (such as erythromycin) should be the priority target for antibiotic stewardship programs to optimize use.[10] The reserve group includes the “last resort” antibiotics, such as meropenem, reserved for treating infections because of multidrug-resistant organisms.[11] Failure of antibiotics to treat effectively an infection in the mouth or elsewhere in the body (e.g., respiratory tract infection) or to provide prophylaxis before major surgery (e.g., joint replacement) can pose a life-threatening risk.[12] For patients with dental infections, the spread of infection toward vital structures in the head and neck may occur rapidly. Optimizing antibiotic use by prescribing guidelines will improve everyone’s outcomes, especially for the most vulnerable.[13] Dental infections are generally amenable to treatment by a dental procedure (such as tooth extraction) to remove the source of the infection without giving antibiotics. In the absence of infection, antibiotics are never appropriate for pain such as that associated with irreversible pulpitis.[14] Dentists are surgeons skilled and equipped to diagnose and treat acute dental conditions during urgent appointments; access to dental, rather than medical, care for patients with acute dental conditions is essential. This growing problem of care provided in non-dental settings (such as hospital emergency departments) contributes to the overuse of antibiotics because the treatment provided is rarely definitive.[15] Guidelines based on these principles while considering other relevant factors (such as antibiotic resistance patterns and access to high-quality antibiotics) sit at the heart of efforts to optimize antibiotic prescribing.[16]
This questionnaire, which was developed based on guidelines provided by the WHO and the Indian Council of Medical Research, underscores dentists’ pivotal role in the fight against antibiotic resistance. These guidelines, designed to optimize antibiotic prescribing and improve patient outcomes, are crucial for dentists to adhere to.
Antibiotics should be prescribed in dentistry only when localized symptoms spread and cause symptoms like fever more significant than 35°C and cellulitis. However, for question 1, most participants, 59.6%, answered that they prescribe antibiotics to the symptoms subside. Most mild infections subside even if bacteria are frequently self-limiting and do not help reduce the occurrence of life-threatening illness.[17]
Regarding the most frequent cause of prescribing antibiotics, 58% of participants answered peri apical abscess followed by pulpitis, periodontal abscess, cellulitis, and pericoronitis. However, abscesses with systemic involvement, cellulitis, and pericoronitis with systemic involvement are indicated for antibiotic use.[18] 73.2 % of the dentists answered that dental extraction is the most common treatment. They prescribe antibiotics, followed by root canal therapy, periodontal surgery, periapical surgery, implant surgery, oral prophylaxis, and root planning. However, previous studies have shown that most dental procedures do not require per-treatment antibiotic use or prevent the occurrence of complications.[19] For the question course of antibiotic therapy, 48.4% of participants answered for 5 days, followed by 3 days and 7 days till the symptoms subside, but in most cases, 3 days are sufficient, followed by 5 days. The most common side effect observed was gastritis, followed by nausea, diarrhea, vomiting, and allergy. For the question, do the dentists know what antibiotic resistance is? 96.8% of participants answered yes, but only 46.4% knew that antibiotic-resistant organisms transfer from person to person, animal to person, and vice versa. Diseases caused by microorganisms resistant to antibiotics are challenging to treat. 82.4 % of participants were aware of this, which might be life-threatening and require the development of newer antibiotics. Antibiotics that were prescribed wrongly can also become a potent factor in increasing the resistance of bacteria.[20] Treatment indication, choice of agent, and duration of antibiotic therapy are incorrect in 30–50% of cases. 30% to 60% of antibiotics prescribed in intensive care units are unnecessary and inappropriate. Incorrectly prescribed antibiotics have questionable therapeutic benefits and expose the patients to potential complications of antibiotic therapy. Sub-inhibitory and sub-therapeutic antibiotic concentrations can promote the development of antibiotic resistance by supporting genetic alterations, such as changes in gene expressions, horizontal gene transfer, and mutagenesis.[21] Up to 10% of antibiotics prescribed in outpatient settings can be by dentists to treat oral and dental infections or prophylaxis of surgical procedures, of which a significant portion is unnecessary or inappropriate.[22]
For the question, main source consulted to obtain information on AMR and antibiotic administration; 37.2% of general practitioners, 33.3% of interns, 27.6% of postgraduates, and 23.2% of specialty practitioners answered that they would consult a textbook, 9.3% of general practitioners, 19.4% of interns, 24.1% of postgraduates, 23.2% of specialty doctors consulted guidelines to prescribe antibiotics. 25.6% of general practitioners, 23.7% of interns, 27.6% of postgraduates, and 19.6% of specialists’ practitioners consulted information from pharmaceutical guidelines. The main reason antibiotics are prescribed without indications is that 55.4% of the specialist practitioners, 43.1% of postgraduates, 39.8% of interns, and 27.9% of general practitioners answered to avoid the occurrence of flare-ups and emergency visits. 37.2% of general practitioners, 29.0% of interns, 36.2% of postgraduates, and 33.9% of specialized practitioners prescribed antibiotics to reduce patient discomfort. Antibiotics are appropriate for inflammatory conditions, including periodontitis, irreversible pulpitis, and dry socket treatment because they cannot prevent severe complications and replace local surgical or non-surgical treatment.[23,24]
Antibiotic prophylaxis is not needed in healthy, immunocompetent patients. It is contraindicated in severely immunocompromised patients, patients with prosthetic heart valves, patients with replaced joints, and patients with rheumatic fever. Dentists need to remember these indications before prescribing any drugs.
CONCLUSION
The study was conducted among dental house surgeons, postgraduate students, and dentists with varied experience levels, specializations, and gender distributions. After reviewing the study results, a few recommendations were suggested: Dental extraction is the most frequent therapy requiring antibiotic intervention (73.2%). The prescribing behavior analysis reveals a predominant trend of prescribing antibiotics until symptoms subside (59.6%), with periapical abscess being the most common reason (58%). There is no need to extend the duration of antibiotic prescription till the symptoms subside entirely, so it is recommended with a notable preference for a 5-day regimen (48.4%). Antibiotic choices predominantly favor amoxicillin and clavulanic acid (56.8%), and cephalosporins are the preferred alternative for those with penicillin allergies (36%).
The surveyed practitioners exhibit a high awareness of antibiotic resistance (96.8%), with a significant proportion acknowledging a connection between prescribing behavior and the development of antibiotic resistance (62.8%). The consultation of guidelines for antibiotic prescriptions, like Chemistry, Manufacturing and Controls guidelines, must be circulated within the practitioner’s community. Various sources, including textbooks (30.4%) and pharmaceutical guidelines (24%), are also available, which can contribute to their knowledge of AMR. In prescribing antibiotics without clear indications, the primary motivations are to avoid complications (42%) and patient discomfort (33.2%).
Furthermore, antibiotic prophylaxis is notably standard for patients with prosthetic heart valves (55.56%). Although the participants know guidelines for prescribing antibiotics in a clinical scenario, they opt for higher antibiotics for longer than first-choice drugs out of fear of flare-ups, to avoid an emergency visit, or to increase patient comfort.
Ethical approval
The research/study approved by the Institutional Review Board at Institute Ethical Committee Narayana Dental College and Hospital (IEC\NDCH), number IEC/NDCH/2023/AUG-SEPT/P60, dated 09th September, 2023.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: This study was financially supported by YSRajasekhar Reddy (YSR) University student short-term research grant 2023.
References
- Global action plan on antimicrobial resistance. 2015. Geneva Switzerland: WHO; Available from: https://fctc.who.int/resources/publications/i/item/9789241509763# [Last accessed on 2024 Aug 15]
- [Google Scholar]
- How did COVID-19 impact on dental antibiotic prescribing across England? Br Dent J. 2020;229:601-4.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic prescribing in UK general dental practice: A cross-sectional study. Community Dent Oral Epidemiol. 2016;44:145-53.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the appropriateness of antibiotic prescriptions for infection prophylaxis before dental procedures, 2011 to 2015. JAMA Netw Open. 2019;2:e193909.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.educba.com/sample-size-formula [Last accessed on 2024 Jul 18]
- Oral microflora and selection of resistance after a single dose of amoxicillin. Clin Microbiol Infect. 2016;22:949.e1-4.
- [CrossRef] [PubMed] [Google Scholar]
- A review of the literature: antibiotic usage and its relevance to the infection in periodontal flaps. Acta Odontol Scand. 2017;75:288-93.
- [CrossRef] [PubMed] [Google Scholar]
- Prophylactic antibiotics for staged bone augmentation in implant dentistry. Acta Odontol Scand. 2020;78:64-73.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic prophylaxis habits in oral implant surgery among dentists in Italy: A cross-sectional survey. BMC Oral Health. 2019;19:265. 10.1186/s12903-019-0981-4
- [CrossRef] [Google Scholar]
- Prescription of antibiotics for pulpal and periapical pathology among dentists in southern Saudi Arabia. J Glob Antimicrob Resist. 2017;9:82-4.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic prescribing practices of dentists for endodontic infections; a cross-sectional study. PLoS One. 2020;15:e0244585.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic and opioid analgesic prescribing patterns of dentists in Vancouver and endodontic specialists in British Columbia. J Can Dent Assoc. 2017;83:h8.
- [Google Scholar]
- Antibiotic and analgesic prescription patterns among dentists or management of dental pain and infection during endodontic treatment. Med Princ Pract. 2018;27:66-72.
- [CrossRef] [PubMed] [Google Scholar]
- The Pattern of antibiotic prescribing by dental practitioners in Zagreb, Croatia. Cent Eur J Public Health. 2015;23:107-13.
- [CrossRef] [PubMed] [Google Scholar]
- Medication prescribing practices in croatian dental offices and their contribution to national consumption. Int Dent J. 2021;71:484-90.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic use for treating dental infections in children: A survey of dentists' prescribing practices. J Am Dent Assoc. 2012;143:31-8.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in dentists' prescribing patterns in Norway 2005-2015. Int Dent J. 2022;72:552-8.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic prescribing by general dentists in the United States, 2013. J Am Dent Assoc. 2017;148:172-8.e1.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic duration and outcome complications for surgical site infection prevention in traumatic mandible fracture. J Surg Res. 2020;247:524-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of prolonged vs short courses of antibiotic prophylaxis following ear, nose, throat, and oral and maxillofacial surgery: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2019;145:610-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal-and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J Am Dent Assoc. 2019;150:906-21.e12.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotics in the treatment of periodontitis. Dent Update. 2004;31:448-50. 453-4, 456
- [CrossRef] [PubMed] [Google Scholar]
- A Review of evidence-based recommendations for pericoronitis management and a systematic review of antibiotic prescribing for pericoronitis among dentists: Inappropriate pericoronitis treatment is a critical factor of antibiotic overuse in dentistry. Int J Environ Res Public Health. 2021;18:6796.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic resistance in general dental practice--a cause for concern? J Antimicrob Chemother. 2004;53:567-76.
- [CrossRef] [PubMed] [Google Scholar]






