Translate this page into:
Oral Mesenchymal Stem Cells: A Budding Branch in Dentistree?
-
Received: ,
Accepted: ,
This article was originally published by Informatics Publishing and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The stem cells possess various possibilities in the regeneration of organs and tissues, thereby helping in the restoration of their normal function. Various types of stem cells and their characteristics such as source, function, limitations and their applications are discussed in detail in this article. Dental and Non-Dental Oral MSC (Mesenchymal Stem Cells) are being compared based on their proliferation ability and regenerative capacity. This article also depicts the regenerative applications of oral mesenchymal stem cells.
1. Introduction
Stem cells are the reservoir cells responsible for the formation of every tissue/organ of the body. They are undifferentiated cells which upon an inductive stimulus undergo mitosis to produce more stem cells and in the presence of various signaling molecules, they can get differentiated into multiple cell lineages. Among the different types of stem cells, Oral Mesenchymal Stem Cells can be easily obtained, cryopreserved, cultured and can be induced to transdifferentiate into any type of specialized cells. Using cell recombination technology by tissue engineering, these transdifferentiated cells can be used to regenerate tissues/organs1,2.
2. Types of Stem Cells1,3–6
Embryonic Stem Cells (ESC).
-
Adult Stem Cells (ASC)
Haematopoietic Stem Cells.
Non- Haematopoietic Stem Cells / Mesenchymal stem cells.
Neural stem cells.
Epithelial stem cells.
Induced Pluripotent Stem Cells (iPS).
Mesenchymal Stem Cells, a type of adult stem cells can be further classified into:
Bone Marrow Stromal Cells (BMSC).
-
Oral Mesenchymal Stem Cells (MSC).
Dental MSCs
Dental Pulp Stem Cells (DPSC).
Stem cells from Human Exfoliated Teeth (SHED).
Stem Cells from Apical Papilla (SCAP).
-
Non-Dental Oral MSCs
Periodontal Ligament Stem Cells (PDLSC).
Dental Follicle Precursor Cells (DFPC).
Skeletal stem cells.
Umbilical Cord Stem Cells.
Menstrual Blood Stem Cells.
3. Embryonic Stem Cells
They are pluripotent stem cells with the ability to differentiate into all the three germ layers namely ectoderm, endoderm and mesoderm.
They are obtained from 2-11 days old embryos which are also known as blastocysts.
-
Limitations
Tumorigenesis(teratoma formation) when transplanted.
Ethical and legal issues regarding use of embryos.
Allogenic immune rejection.
-
Applications in Dentistry
Regeneration of mucosa, alveolar bone and periodontal ligament.
-
Applications in Regenerative Medicine
4. Adult Stem Cells
These stem cells can differentiate into different cell types of their tissue of origin and also can transdifferentiate into different cell lineages thereby helping in the repair of the injured tissues.
These stem cells are present in many organs and tissues such as: brain, bone marrow, peripheral blood, skeletal muscles, skin, teeth, heart, gut, liver, pancreas, fat, retina, testis and ovarian epithelium.
Their capacity of broad cellular differentiation is dependent on and responsive to specific cues present in the engrafted site environment6.
They reside in a specific area of each tissue called as the stem cell niche and its isolation from human body is challenging. Once these stem cells are removed from the body, their capacity to divide in that tissue becomes limited thus making it difficult to harvest them in large quantities.
Among the Adult stem cells, oral MSCs, Umbilical Cord Stem Cells (UC-SC) and Menstrual Blood Stem Cells (MB-SC) are easily accessible and can be harvested conveniently. They exhibit excellent proliferation and differentiation abilities and are less susceptible to contamination by bacteria and virus5.
Adult stem cells are less likely to be rejected after transplantation because they can be isolated from same patient8.
5. Induced Pluripotent Stem Cells
These are Adult Stem Cells that are reprogrammed genetically to become an embryonic stem cell like state with the ability to produce all the three germ layers.
iPS cells can be obtained from many types of oral tissues like SHED, SCAP, DPSC, tooth germ progenitor cells(TGPC), buccal mucosa fibroblasts, gingival fibroblasts, and PDL fibroblasts.
In contrast to adult stem cells, iPS cells provide an unlimited source of cells for clinical applications.
In contrast to ESC, iPS cells are autologous and patient specific without any ethical issues.
The ability of iPS cells to form tooth-like structures in vivo and periodontal tissue regeneration (cementum, alveolar bone and PDL) has been confirmed by different studies3,7.
6. Oral Mesenchymal Stem Cells
Oral MSCs have the general characteristics of stem cells like self-renewal, multipotency and in- vivo tissue regeneration capacity. They are far more superior to human Bone Marrow derived MSC (BMMSC) because
Oral MSCs express higher telomerase activity than BMMSC.
They have higher proliferation ability than BMMSC.
Their CFU (Colony Forming Unit)-F forming ability is five times more than BMMSC9.
6.1 DENTAL MSCs
They are stem cells that are capable of producing dentin pulp complex in-vivo4.
6.1.1 Dental Pulp Stem Cells (DPSC)
They are the first oral mesenchymal stem cells obtained from the permanent third molars7.
They have higher proliferation ability and show three times the population doubling scores than BMMSC9.
DPSC can produce different colonies of multiple cell lineages in the presence of various growth factors, receptors and signaling molecules.
DPSC can differentiate into odontoblast, osteoblast, chondrocyte, myocyte, neurocyte, adipocyte, corneal epithelial cell and melanoma cell.
They are useful in the regeneration of dentin-pulp complex, neuro and gliogenesis in vivo7.
6.1.2 Stem Cells from Human Exfoliated Deciduous Teeth (SHED)
SHED obtained from the pulp of human exfoliated deciduous teeth, show four times population doubling score than the BMMSC and thus have higher proliferation rate than BMMSC and DPSC9.
They are the ideal source of oral MSCs with the ability to regenerate complete dentin/pulp complex and also with osteoinductive capacity in vivo3.
They can repair calvarial defects in animals and are also beneficial in treatment of neurodegenerative diseases.
Both DPSC & SHED express neural markers7.
6.1.3 Stem Cells from Apical Papilla (SCAP)
SCAP are obtained from the loosely attached tissue at the apex of the developing permanent teeth.
SCAP residing in apical papilla is responsible for apexogenesis of immature permanent teeth with apical periodontitis and abscess.
They have high proliferation activity in vitro compared to DPSC, thus being useful in cell regeneration and root formation.
Helps in dentin production in vivo and formation of root dentin.
They also can differentiate into osteoblast, adipocyte, chondrocyte, and neuron under specific conditions7.
6.2 Non- Dental Oral MSCs
They are oral MSCs that are not capable of producing dentin-pulp complex4.
6.2.1 Periodontal Ligament Stem Cells (PDLSC)
Human PDLSCs can be isolated from root of extracted teeth.
They have the potential for regeneration of cementum and PDL like structure.
They also have a limited ability of differentiation into osteogenic tissue.
They express cell surface marker similar to BMMSC and DPSC.
PDLSC are used to treat periodontitis and can be used to create a biological root in implants7.
6.2.2 Dental Follicle Precursor Cells (DFPC)
Dental Follicle Precursor Cells (DFPC) is obtained from loose connective tissue surrounding the developing tooth.
They have high proliferative activity and can be used for cementum, bone and PDL regeneration.
They possess the potential to form calcified nodules in vitro.
DFPC exhibit highest plasticity among all the other oral MSC and have a high proliferative activity7.
7. Oral MSC Applications
It has been suggested that the oral MSCs are potential source of stem cells for orthopedic, oral, and maxillofacial reconstruction. Interestingly, the general procedure for human oral MSC administration is to implant them in scaffold or porous biomaterial to reinforce the graft site and induce tissue regeneration.
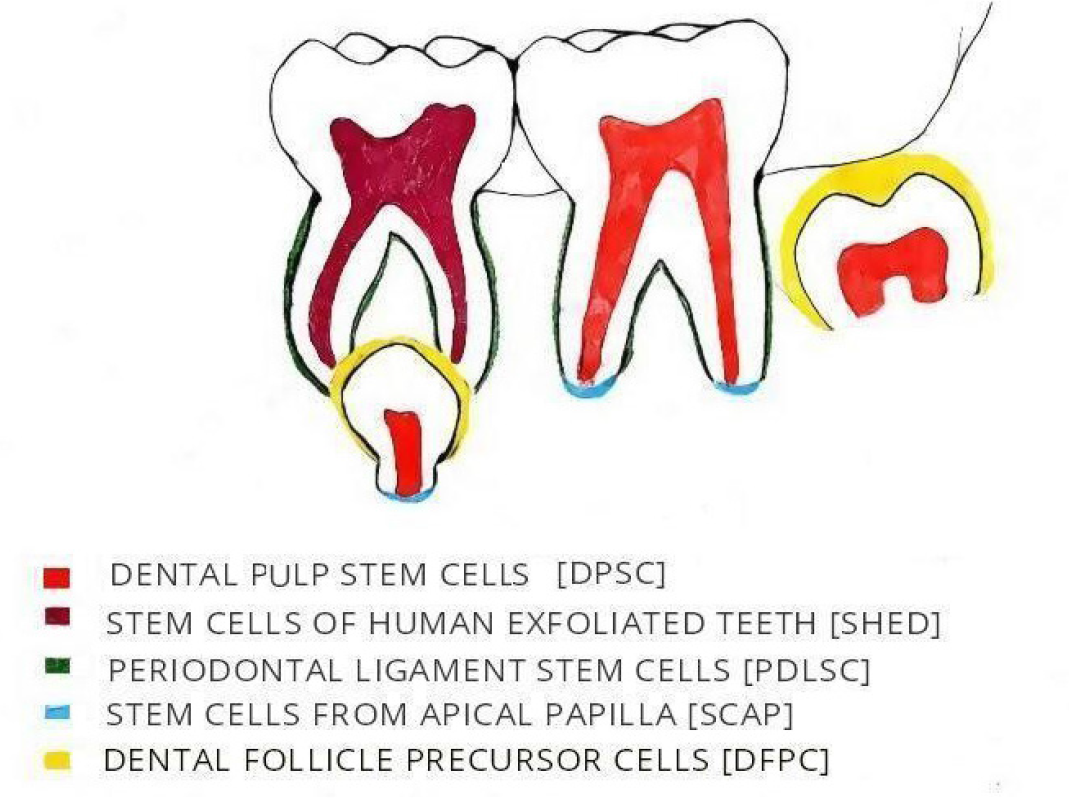
- Source of Oral Mesenchymal Stem Cells.
8. Regenerative Applications of Oral MSCS
8.1 Dentin-Pulp Regeneration
DPSC has the potential to produce dentine pulp like complex both in vitro and in vivo after subcutaneous transplantation. DPSC along with SHED/ SCAP can regenerate pulp like tissue in emptied root canal space with >2mm in diameter after subcutaneous transplantation. When stimulated by the calcific carriers, the dental MSC can regenerate dental pulp4. DPSC/SCAP implanted in injectable bio-active scaffold with various signaling molecules when placed within disinfected root canal of mature dog teeth with enlarged apical foramen showed complete regeneration and revascularization of dental pulp (in situ). This procedure called cell homing for pulp regeneration uses SDF - 1α (Stromal Derived Factor) loaded silk fibroin scaffold10.
8.2 Periodontal Regeneration
PDLSC/DFPC plays a major role in periodontal regeneration. They promote bone regeneration, inhibit inflammation and prevent tooth loss thereby preserving the vitality of the tooth. They enable healing of the periodontium in dehiscence defects of dogs by bone, PDL and cementum formation. Vitamin C treatment in PDLSC induces the telomerase activity within them thereby improving their periodontal regeneration4.
8.3 Bone Regeneration
Oral MSC under suitable environment can differentiate into osteoblasts and produce bone11. The subpopulation of DPSC which differentiate into osteoblasts are referred to as Stromal Bone Producing (SBP) DPSC12. Osteoblasts derived from oral MSCs are similar to normal primary osteoblasts both functionally and phenotypically, but differ in their gene expression profiles2. DPSC, PDLSC and SHED exhibit bone regeneration among which DPSC and PDLSC showed superior efficiency compared to BMSC in bone regeneration11. Several studies showed formation of woven bone chips with an integral blood supply in immunocompromised (IC) animals. Osseous regeneration of DPSC around titanium dental implants exhibited highest osteogenic potential than BMSC. A clinical trial conducted to repair mandibular bone defects with DPSC showed positive clinical outcome with few limitations to use DPSC for bone regeneration at clinical level2.
8.4 Neural Regeneration
DPSC when compared to BMSC have higher differentiation efficiency to form neural cells. A significant subpopulation of DPSC upon stimulation in neural differentiation media express several neural markers, formation of neurospheres invitro and possess effective neuronal induction. Transplanted DPSC promote repair and regeneration of the injured peripheral nerves by secreting neurotrophic factors and provoking a chain reaction in the neighbouring cells to differentiate and secrete neurotrophic factors4,11.
8.5 Complete Dental Regeneration
Complete functional tooth regeneration was made possible by combining mouse dental epithelium and oral MSCs. The main limitation for bioengineering a human tooth is the isolation of the human epithelial stem cells which can be overcome by using iPS cells2,3.
8.6 Muscle Regeneration
DPSC has the ability to differentiate into cardiomyocyte like cells invitro on cocultivation with neonatal cardiomyocytes and into dystropin-producing muscle cells in cardiotoxin-paralyzed muscles in mouse model. This can be applied in treatment of muscular dystrophy4.
8.7 Tendon and Cartilage Regeneration
Subcutaneous transplantation of PDLSC in immuno-compromised (IC) mice forms tendon like tissue which is more organized with increased extracellular matrix and collagen than those produced by the GMSC (Gingival Mesenchymal Stem Cells) and BMSC. Invitro chondrogenic induction of PDLSC and GMSC can differentiate into chondrocyte like cells upon chondrogenic induction thereby showing potential for cartilage regeneration4.
8.8 Salivary Gland Regeneration
DPSC along with human salivary gland cell lines in either Matrigel or Hyaluronic acid hydrogel scaffolds generate salisphere like structures. These structures express markers of acinar cells and upon subcutaneous transplantation in IC mice, generate a well irrigated salivary gland like tissue. During invitro recombination of embryonic mouse salivary epithelia and human DPSC in 3D- Laminin or extracellular matrix scaffold, DPSC bind and interact with the salivary epithelia and also improved the invitro viability of the tissue. The role of DPSC in salivary gland regeneration is to generate the salivary stroma (mesenchymal component) because the oral MSC are neural crest derived cells2.
9. Conclusion
Oral MSC are evolving to be most beneficial in the budding branch of dentistree. They indeed play a beneficial role in the regeneration of dentine, pulp, periodontium, bone, muscles, tendon, cartilage and salivary gland. It is also advantage that the oral MSCs possess higher proliferation rate than the other stem cells.
Despite the fact that the oral MSCs are easily obtained from discarded biological materials, their higher proliferative ability and regeneration of injured tissues, their clinical applications is yet to be fully tapped. From this article, we understand that the oral mesenchymal stem cells have emerged to be a promising future in tissue engineering (Regenerative Dentistry).
References
- Stem cells: An emerging future in dentistry. International Journal of Advanced Health Sciences. 2014;1(2):17-23.
- [Google Scholar]
- Dental pulp stem cells as a multifaceted tool for bioengineering and the regeneration of craniomaxillofacial tissues. Frontiers in Physiology. 2015;6:1-10.
- [Google Scholar]
- Stem cell sources for tooth regeneration: Current status and future prospects. Frontiers in Physiology. 2014;5(36):1-10.
- [Google Scholar]
- From regenerative dentistry to regenerative medicine: Progress, challenges and potential applications of oral stem cells. Stem Cells and Cloning: Advances and Applications. 2014;7:89-99.
- [Google Scholar]
- Comparative analysis of human mesenchymal stem cells from the umbilical cord, dental pulp and menstrual blood as sources for cell therapy. Stem Cells International 2016:1-13.
- [Google Scholar]
- Origin and use of embryonic and adult stem cells in differentiation and tissue repair. Cardiovascular Research. 2003;58:324-35.
- [Google Scholar]
- Mesenchymal stem cells in dental tissues: perspectives for tissue regeneration. Braz Dent J. 2011;22(2):91-8.
- [Google Scholar]
- Therapeutic potential of stem cells in regenerative dentistry: A review of literature. International Dental Journal of Student’s Research. 2013;1(4):22-30.
- [Google Scholar]
- Properties and possibilities of human dental pulp-derived stem cells. Archives of Stem Cell Research. 2015;2(2):1012:1-6.
- [Google Scholar]
- Pulp regeneration: Current approaches and future challenges. Frontiers in Physiology. 2016;7(58):1-8.
- [Google Scholar]
- Regenerative applications using tooth derived stem cells in other than tooth regeneration: A literature review. Stem Cells International 2016:1-12.
- [Google Scholar]
- Dental pulp stem cells: function, isolation and applications in regenerative medicine. Journal of Tissue Engineering and Regenerative Medicine. 2015;9:1205-16.
- [Google Scholar]





