Translate this page into:
Lab-on-a-chip – The advent of instantaneous diagnosis for a plethora of diseases

*Corresponding author Gayathri Sanjay, House Surgeon, Department of Oral Medicine and Radiology, SDM College of Dental Sciences and Hospital, Shri Dharmasthala Manjunatheshwara University, Dharwad, Karnataka, India. gayathrisanjay3@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sanjay G, Shreedhara L, Mallya V, Sarpangala P, Guttal K, Nandimath K. Lab-on-a-chip – The advent of instantaneous diagnosis for a plethora of diseases. J Academy Dent Educ. 2023;9:64-72. doi: 10.25259/JADE_30_2023
Abstract
A lab-on-a-chip (LOC) is a device that facilitates the incorporation of a concatenation of various laboratory functions on a unique integrated circuit. The size of this instrument is merely a few millimeters to centimeters designed to attain automation and high-throughput screening. Microfluidic systems used in LOC devices allow the manufacture of millions of microchannels, each measuring mere micrometers. These microchannels enable control of fluids in infinitesimal quantities for a variety of diagnoses. Several labs on a chip have been commercialized in recent times for fundamental procedures, including glucose monitoring, human immunodeficiency virus, early tumor detection, and cardiac diagnostics. The LOC integrates microfluidics, nanosensors, micro-electrics, and biochemistry on one device. The advantages of the chip include its sustainability and cutback wastage. It expedites a decline in reagent costs and requires minimal sample volumes. The analysis and response are faster and the response is better controlled by equipping micro-channels. Countries with exiguous healthcare are in the face of adversity due to increased fatality rates from infectious diseases that are often curable in developed nations. In certain circumstances, impoverished healthcare clinics have the medications requisite to treat a specific condition but are in dearth of the diagnostic equipment needed to determine, in which individuals are in need of the medications. This is where the role of LOC as a potent novel diagnostic instrument would benefit humankind in the nearest future, according to eminent researchers. This article highlights the applications of LOC in a miscellany of fields, its advantages, feasible means to overcome the drawbacks, and the propitious prospects of this technology.
Keywords
Microfluidic devices
Nanosensors
Micro-channels
High-throughput screening
Integrated circuit
INTRODUCTION
Most of the underdeveloped and developing nations have a substantial population residing in remote areas. Such regions suffer from deadly diseases due to a dearth of resources, awareness, and affordability. An abundance of drug supply would not suffice if it does not reach the suitable patients. Thereby, point-of-care (POC) diagnostics is the long-term solution for timely, remote, and rapid diagnosis to reduce fatality from such diseases.[1]
A gadget called a “lab-on-a-chip” (LOC) incorporates numerous operations, routinely performed in a laboratory, such as chemical synthesis and analysis, in a chip format, that measures a few millimeters to a few square centimeters varying based on the application.[2] The size of the microfluidic channels typically ranges from 100 micrometers (mm) and 1 mm in diameter. It associates several techniques such as fluidics, electronics, optics, and biosensors [Figure 1].[3]
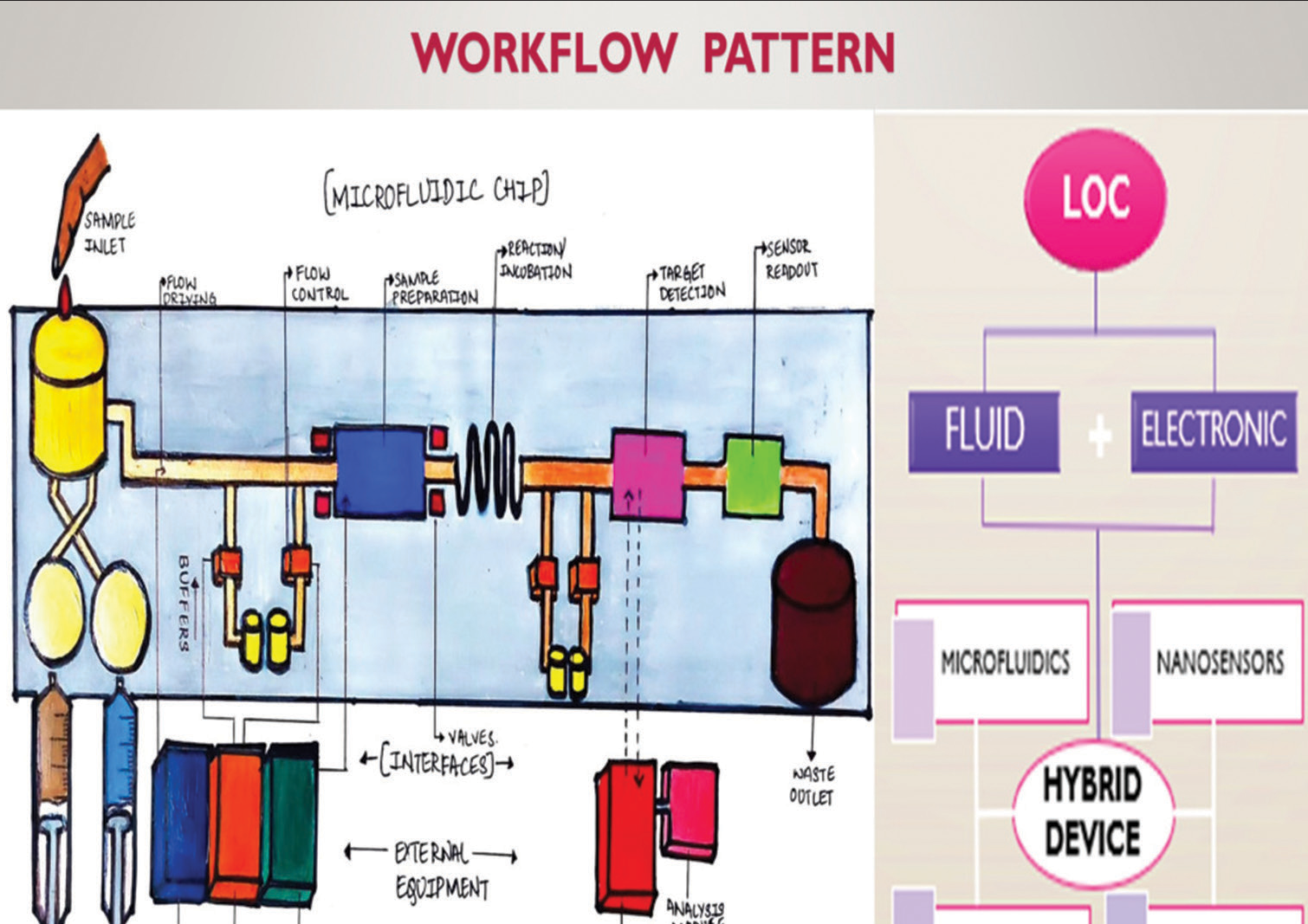
- Working principle of lab-on-a-chip technology.
Photolithography is the most widely used fabrication technique. For a speedy and cost-efficient manufacturing process, polydimethylsiloxane (PDMS) microfluidic devices were introduced. 3D printing is currently the most popular method for chip fabrication.[4]
LOC technology is predicated on the scientific realm of microfluidics, which deals with the behavior, manipulation, and control of fluids. Microfluidics is an analysis of the dynamics of fluids across micro-channels and the creation of microscopic devices having micro-channels through which liquids flow.
The working of LOC modality necessitates the amalgamation of expertise from multi-faceted disciplines of engineering, research prodigies of chemistry, physics, and biology, along with medical professionals as it includes intensive designing of materials, separation of materials using microfluidics, sample preparation, detection approaches, etc. Applications of LOC encompass medical diagnostics, environmental monitoring, food quality control, early detection of warfare chemicals, and drug discovery, in furtherance of continuing efforts to broaden knowledge of fundamental concepts (like transport through nanofluidic channels) and refine the LOC toolkit.
It is expected that the adoption of LOC will have various advantages over present test methodologies. The most crucial are quick diagnostics at the POC and modest amounts of samples and supplies necessary to complete testing. However, attention to quality management factors such as device calibration and maintenance, as well as user training and education, are required. As a result, the benefits of LOC applications will not jeopardize the quality of health care or patient safety.
Acknowledging the fact that general practitioners may do tests instantly or even the patients themselves, utilization of LOC applications will contribute to the prevailing trend of greater self-reliance in health care. Furthermore, LOC technology will aid in the creation of medicines tailored to the demands of the patient (personalized medicine). The deployment of LOC applications by health-care professionals is going to escalate if they are operating in the design and development of new devices that match their demands.[5]
LOCs are now developed as monolithic devices which analyze samples by ratifying them through a pre-set sequence of elements coupled by fixed and preconfigured microfluidic channels. To improve the sustainability, efficacy, and versatility of LOCs, networking functions that allow the sequence of items engaged in the processing to be autonomously adopted can be implemented.
Microfluidic paper-based analytical devices (PADs) have been created in massive quantities. In contrast to conventional glucose test strips, PADs improve reagent efficiency and control and enable simultaneous measurement of multiple analytes using hydrophobic barriers in hydrophilic paper to restrict fluid flow to a selected zone. Along with several common techniques for PAD manufacture, such as wax printing, photolithography, and screen printing, other simpler procedures were developed. For instance, Garcia et al. produced the gadget using a portable metal stamp.
In a nutshell, paraffinized paper was utilized to cover native paper. The design in the metal stamp was transferred to the native paper by heating the metal stamp and applying pressure to the paraffinized paper. The total cost of a single PAD came to just $4. In addition to glucose, their approach also identified nitrite, bovine serum albumin, and uric acid. Likewise chemically altering the paper surface to bind enzymes covalently boost color homogeneity inside the sensor area, considerably reducing test variation. All four analytes in the semi-quantitative measurements of the artificial urine samples had an error of <4%. The hydrophobic zones were created by Oyola-Reynoso et al. using a ballpoint pen and silane/hexane ink.[6]
The arrangement of novel nanomaterials with PAD, which enhanced the detection signal, was also reported by the researchers. Silica nanoparticles were incorporated into the device by Evans et al. to increase the strength and constancy of the color signal. This method resulted in a glucose LOD of 0.5 mM. Gold nanoparticles were blended with PAD by Palazzo et al. for colorimetric glucose testing. With this method, color bleaching – which happens with conventional bio enzymatic devices – is eliminated. Many PADs used the electrochemical method in addition to the colorimetric detection methodology to detect glucose with high sensitivity, either by directly printing the electrodes on paper substrates or combining commercially available screen-printed electrodes with the PAD. Since signal detection of PAD-based technologies is very compatible with conventional glucose meters, existing signal readers can be readily modified.
Integrating an array of laboratory processes or one procedure onto an opus miniaturized chip is the ultimate goal of LOC. On just one module, LOC has the capacity to perform any laboratory test, including deoxyribonucleic acid (DNA) sequencing, biochemical detection, synthesis of chemicals, clinical diagnostics, and recognition of biomarkers.
Aim
The aim of this study was to provide an overview of the functioning, applications, and potential advantages and possibilities given by each approach and to envisage the future developments of microfluidic chip technology.
METHODOLOGY
The existing articles in the PubMed database have been referred to using the keywords LOC, microfluidic technology, organ-on-a-chip, microfluidic devices, nanosensors, micro-channels, high-throughput screening, and integrated circuit.
HISTORY
The breakthrough in microtechnology (1954) leads to various applications of semiconductor chips in microelectronic chips. They were predominantly lithography-based technologies equipped for pressure sensor manufacturing, sensors for airbags, and supplementary constituents which are mechanically mobile.
Of late, it has been industrialized as fluid-controlling devices, principally channels, mixers, valves, pumps, and dosing equipment.
S.C. Terry of Stanford University fabricated the LOC analysis system for the 1st time ever, as a gas chromatograph, in 1979. LOC research did not begin to flourish until the tail end of the 1980s and the beginning of the 1990s. Micropumps and flow sensors were developed by substantial research organizations in Europe for integrated fluid analysis and treatment systems. This exemplified that the conglomeration of pre-treatment activities, typically conducted at a lab scale, may expand the basic sensor functioning toward a thorough laboratory examination, including additional cleaning and separation steps.[7]
A revolutionary upswing in both scientific and commercial interest was observed midway through the 1900s, when microtechnology offered remarkable instrumentation for genomics applications, namely, capillary electrophoresis and DNA microarrays. The military has made commendable progress in its research assistance, particularly from the Defense Advanced Research Projects Agency (DARPA), which is interested in portable systems for detecting biological and chemical warfare agents.[8] The application was not just limited to the integration of lab procedures for analysis; it could also be used to extend the capabilities of individual components to other lab operations that were not analysis-related. Consequently, the phrase “lab-on-a-chip” was coined.
APPLICATIONS
POC testing
The widely-known and easily available at-home pregnancy test kit is one of the most acclaimed LOC devices to reach the market. This tool utilizes paper-based microfluidics technology to detect human chorionic gonadotropin levels and yield immediate results. The diagnosis and treatment of prevalent infectious disorders brought on by viruses such as influenza or bacteria like bacteriuria is another prominent field of LOC research.[9] Microbial cell culture is the gold standard for bacteriuria confirmation. A microbiological culture was recently downsized into a dipstick format for the use at the point of care in a study using LOC technology, called Digital Dipstick.
To diagnose malaria, the LabChip real-time polymerase chain reaction (LRP) employed LOC microfluidic technology. A study was conducted to assess and evaluate the diagnostic capacity of LRPs to detect malarial parasites. Two hundred and thirteen people with illnesses and 150 healthy people were enrolled between May 2009 and October 2015. The typical sensitivity of reverse transcription-polymerase chain reaction (RT-PCR) was compared to the diagnostic sensitivity of LRP for malaria parasites. For Plasmodium vivax, Plasmodium falciparum, Plasmodium malariae, and Plasmodium ovale, respectively, the sensitivity of LRP was 95.5%, 96.0%, 100%, and 100%. For P. vivax, P. falciparum, P. malariae, and P. ovale, respectively, the specificity of LRP was 100%, 99.3%, 100%, and 100%. The Cohen’s Kappa coefficients between LRP and CFX96 for the identification of P. vivax, P. falciparum, P. malariae, and P. ovale were 0.96, 0.98, 1.00, and 1.00, respectively. Traditional RTPCR, microscopic analysis, and LRP results did not significantly differ from one another. DNA amplification using LRP and conventional RT-PCR required 27 and 86 min, respectively. LRP amplified DNAs 2 times quicker than RT-PCR due to a more rapid heat transfer. With 68.7% sensitivity and 80% specificity, this technology was able to identify between patients with initial and end-stage ovarian cancer and healthy controls.
Mughal et al. generated a simple-to-use microfluidic-based fluorescence in situ hybridization (FISH) method to screen for hematological malignancies.[10] The device was produced by incorporating glass slide etching and photolithography. Cells were immobilized and used for FISH research in the microchannels. This equipment can analyze ten samples in a sub-microliter container with a limit of detection of 1:100 cells for minimal residual disease analysis, contrasted to the 1:1000 cells from the normal FISH.
Timely diagnosis of sickle cell anemia
One of the most eagerly anticipated LOC technologies is certainly one that can quickly and accurately identify neonates with sickle cell disease using just a single blood droplet. The tool is a miniature replica of the electrophoresis test that is already available for evaluating the condition in babies. Even in remote, underdeveloped regions without access to contemporary diagnostic testing technology, such as parts of Africa and India, it can be widely used.[11] More than 300,000 infants in these regions are born every year with sickle cell disease, but many of them go untreated and pass away before turning five.[12]
Targeted therapy of lung diseases
This chip, which is currently in its last phases of development, has the potential to enhance the management of lung disorders such as asthma, chronic obstructive pulmonary disease, and others. It gathers tiny lung sputum samples, uses sound waves to break them into smaller cell components, and then labels the smaller cell components with chemical tracers for simple detection.[13,14] Following that, the chip can be examined using flow cytometry, a method that counts and classifies the cell kinds and quantities on a sample using strong lasers. The sort of inflammation that is present in the lungs can be determined with great certainty due to these cells.[15]
Screening for heart toxicity
LOCs, which are essentially tiny replicas of human organs, are the ancestors of the so-called organ-on-a-chip technologies. Heart-on-a-chip technology is an illustration of this. The University of California researchers created multifaceted, pulsing cardiac muscle cells that mimic human heart tissue on an inch-long chip.
The researchers confirmed that their gadget may be used to test a wide range of cardiovascular medications for potential heart effects. The time and money generally required to introduce a new medicine to the market could be greatly reduced by the heart-on-a-chip.[16] During the early rounds of testing, the heart-on-a-chip showed promise in assessing the potential toxicity of COVID-19 medicines.
Remedy for sleep disorders
Sleep problems are the latest domain of LOC research. The development of an implanted device that could regulate the body’s circadian clock and speed up people’s recovery from jet lag has recently been given a multimillion-dollar grant to Northwestern University researchers. The LOC technology, which has the moniker “living pharmacy,” will create identical peptides as the physiological peptides that control the sleep-wake cycle.[17] Soldiers may soon be able to conquer battlefields across time zones thanks to an experimental technology financed primarily by the DARPA.[18]
Deciphering the cure for COVID-19
The assembly of the human airway on a chip is used for quick pharmacological screening for COVID 19 treatment options. This was achieved successfully by Don Ingber, M.D., Ph.D., founding director of the Wyss Institute at Harvard University, and his colleagues. The precision and accuracy of drug testing can be enhanced by replicating the cellular environment of the human lung.
This will also obviate the ruthless practice of animal testing, often associated with high failure rates.
Assessing 38 clinical saliva samples, including 20 samples that tested positive for the Alpha variation (sensitivity > 90%, specificity = 100%), verified the reliability of the designed point-of-care equipment.[19]
The chip exhibited its proficiency in testing anti-SARSCoV-2 and anti-flu medications. Amodiaquine, an antimalarial substance, was subsequently identified as an effective treatment for COVID. Clinical trials at multiple sites in Africa are being conducted.
Saliva as a biomarker
Saliva can be used to assess overall health because it contains several diagnostic substances such as steroid hormones, human immunodeficiency virus (HIV) antibodies, and many other molecular components. Hence, it is a non-invasive and feasible method to study microbes and chemical and immunological markers. It is identified to be as effective as serum in the diagnosis of autoimmune diseases, hereditary disorders, infectious diseases, malignancies, and metabolic disorders. It can also be used to measure the quantity of drugs in the body and it is a potential tool to control drug abuse. It is very easy to collect, cost-effective, and can be used for mass screening as a chair-side procedure.
Hence, it is an excellent tool in diagnostics.
A micro-electro-mechanical device called the oral fluid nanosensor test (OFNASET) or LOC nanotechnology was developed by Gau V and Wong D in collaboration with the University of California, Los Angeles for the detection of liquid biopsy-based biomarkers. The OFNASET is designed to detect salivary biomarkers for oral cancer, including four salivary messengers ribonucleic acid (RNA) biomarkers and two salivary proteome biomarkers (thioredoxin and interleukin-8 [IL-8]) (SAT, ODZ, IL-8, and IL-1b). If cancer is detected early, the mortality and morbidity associated with it can be reduced significantly.[20] The patient can administer LOC themselves, which not only makes it more convenient but also speeds up diagnosis.
Tooth-on-a-chip
Applying biomaterials directly to the cavities that have formed on the tooth surface is mandatory for the management of oral ailments such as hypersensitivity and tooth decay. These usually involve the attachment of biomaterials to the dentin. Thus, the tooth’s structural arrangement generates an extraneous interface where biomaterials interact with the dentin matrix indirectly through dentinal tubules, which then allow reaction by-products and leaching agents to seep into the pulp beneath.[21] These dentinal tubules harbor odontoblastic processes that are uniform throughout the dentin matrix and extend into the tubules of considerable length. This dentin-pulp biomaterial is unique to human corpulence and provides crucial details regarding the propensity of teeth to react to biomaterials.[22] To examine dental pulp cell response to biomaterials, an organ-on-a-chip model system was created. This system simulates some of the architecture and dynamics of the dentin-pulp interface.
The tooth-on-a-chip is devised from molded PDMS, and its structure consists of two chambers partitioned by a layer of dentin. Increased knowledge of the cytotoxicity of these materials was made possible by the illustration of pulp cell reaction in response to dental materials performed on this chip. The apical papilla stem cells were cultured in the odontogenic media. After that, they were propagated on the dentinal surface for live-cell microscopy analysis.[23] Despite its novelty, the tooth-on-a-chip device can offer many benefits in the near future. On average, the endurance of esthetic restorations is about 5–7 years. Although some stay intact longer, old restorations often do not provide satisfactory protection from caries. This means that during a lifetime, an average patient may have to replace restorations several times.[24]
If the tooth-on-a-chip device provides the much-needed insight into the dynamics of the interaction between dentin and pulp and the morphologic, metabolic, and functional influence of biomaterials on dental pulp cells that are viable, there will be further durable teeth repair solutions and higher quality dental work in the future.
Dentists could identify durable restorative materials that are persistent. This would enable them to formulate customized treatment modalities by virtue of patients’ oral microbiome and individual characteristics of their teeth.[25] A tooth-on-a-chip has been examined employing HEMA and 35% phosphoric acid, and it has been discovered that it reproduces the tooth’s nearly physiologic environment and enables live cell imaging to research biomaterials.[26]
The various applications of LOC technology are shown in [Table 1].[27]
| Drug testing and research | Diagnosis | Treatment |
|---|---|---|
| 1. COVID-19- Drug screening for influenza and COVID-19. Amodiaquine proved efficient against COVID-19. 2. Dental biomaterials- HEMA and 35% Phosphoric acid replicates near physiologic conditions of the tooth, enabling live cell imaging to study biomaterials. 3. Heart toxicity- Microscopic versions of organs have been made on chips, for example, like heart-on-a-chip for screening cardiovascular drugs. |
1. Point-of-care testing- Pregnancy test kit, urine test for infectious diseases, digital dipstick. 2. Sickle cell anemia- A drop of blood is used to detect the sickle cell gene in nurslings on a miniaturized electrophoresis testing device 3. Salivary biomarkers- Saliva contains steroid hormones, HIV antibodies, and many other molecular components which is a non-invasive and feasible method of diagnosis. Also used to measure the quantity of drugs in the body |
1. Lung diseases- Treatment of asthma, COPD, etc., using diminutive sputum samples based on the principle of flow cytometry 2. Sleep disorders- The recuperation of jet lag and other sleep disorders encountered by night shift employees is brought about by an implantable device that regulates the body’s circadian rhythm. 3. Tooth-on-a-chip- Offers customized therapy according to the specific individual oral microbiome and tooth characteristics |
COPD: Chronic obstructive pulmonary disease, HIV: Human immunodeficiency virus
Integration of LOC with smartphone devices
Since over a decade ago, there have been home self-diagnostic tools for estimating blood cholesterol, but their widespread use has been constrained by the relatively high cost of purchasing a quantitative test-strip reader, the device’s challenging operation, and the inability to quickly store and process results. A smartphone attachment and software program that measures blood cholesterol levels was developed, as a solution to this problem. In a series of human experiments, the device managed to precisely determine the total cholesterol levels in the blood within 60 s by imaging traditional test strips. Furthermore, the device has been upgraded to improve measurement repeatability and sensitivity across various telephones. Smartphone adoption and the development of more advanced image-processing techniques will greatly improve cardiovascular disease prevention by improving the accuracy and scope of cholesterol monitoring.
Using smartphone-linked microfluidic lab-on-chip technology, living algae in simulated ballast water samples were detected, counted, and measured in real time. The device is composed of a microfluidic chip, a unique fluorescence imaging platform, and a smartphone. When living algae pass through the laser-illuminated portion of the microfluidic chip, chlorophyll fluorescence is released and shown on the smartphone. The spotted living algae are identified, counted, and quantified with a smartphone algorithm. As a proof of concept, two different sizes of algae were tested, and a specificity for detection of more than 92% was achieved. The sizing precision is 94%. The maximum detectable moving velocity is governed by the diameter of the algae and the smartphone’s frame rate (frame per second, fps).[28]
The maximum movement velocity allowed for detection relies on the algae’s diameter and the smartphone’s fps. Due to its straightforward design and operation, the instrument is appropriate for on-site assessments of the living state of the algae Chlorella vulgaris in treated ship bilge water.
A smartphone attachment developed by researchers can rapidly detect several infectious diseases from a drop of blood. A miniature cartridge within the phone has detection zones designed to scan for disease-specific antibodies in the blood.
The researchers pursued this gadget to screen 96 patients for HIV and syphilis at different community clinics in Rwanda during its field testing. The results were 96% as accurate at identifying numerous infections as those from conventional lab tests.
A useful tool for instant diagnoses through mobile clinics in the field, the latest innovation is inexpensive and consumes far less power for detection and result presentation.
Commercialization
The applications for current LOCs have been gathered into an inventory. With 154 devices now on the market and 33 devices in development, it consists of 75 firms. Numerous of these applications involve instruments for measuring cardiac indicators, analyzing blood glucose and electrolytes, and diagnosing HIV. Abbott, Alere, Arkray, Bayer, LifeScan, Menarini Diagnostics, Roche, and Siemens are the market leaders in LOC diagnostics.
For instance, the iSTAT from Abbott Diagnostics quickly analyzes relatively small amounts of blood. LOC technology has been utilized successfully in the detection of analytes such as electrolytes in blood samples. It consists of electrode arrays that have been placed on silicon cartridges to create a biosensor.[29]
Before the analysis, a few droplets of sample blood that are pulled into the cartridge by capillary action are chemically treated. Then, using a portable electromechanical tool, the concentration of electrolytes or other analytes in the blood sample is monitored.[30]
ADVANTAGES
LOCs provide several advantages based on their application. Lower fluid volumes and cost
Reduced turnaround time
High parallelization
Facilitates ease of operation due to its compactness
Minimizes human error[31]
It also decreases the time to acquire results, which makes it more amenable to the point-of-care testing space
Interrogation of a much smaller volume of sample often eliminates the need for sample processing
Accessible to all.
DISADVANTAGES
Micro-fabrication processes of LOC demand trained personnel, expensive equipment, and technique sensitivity. However, it can be solved by 3D printing and laser engraving[32]
Working on a LOC necessitates intricate fluid movements through channels that can be laborious to calibrate. However, this can be avoided by including pumps, using air bag-embedded pumps, or employing centrifugal force
The majority of LOCs are au courant proof-of-concept programs that must obtain endorsement before they can be implemented in practice[33]
The results of LOC can be impacted by capillary forces, surface roughness, or other chemical reactions when rigorously scaled down to microliters. Consequently, when compared to LOC, the processes carried out leveraging traditional laboratory equipment may differ.[34]
THE FUTURE ROADMAP
The processing of samples on an economical and eco-friendly platform for a “sample-to-answer” analysis is one of the main barriers that will need to be overcome in the future for the development of such technologies. In addition to contributing to a reduction in the consequences of pandemics, these novel discoveries will be crucial in addressing the challenges set forth by the World Health Organization (WHO) regarding the eradication of endemic illness (WHO, 2020), particularly in the developing world. This contains several “neglected” illnesses like schistosomiasis in addition to more prevalent illnesses such as malaria and hepatitis.
The development of novel, more precise diagnostics based on molecular testing that can identify asymptomatic and/or presymptomatic patients as well as disease reservoirs in rural and underserved populations offers a significant challenge for the future (Alvar et al., 2020).
The basic prospects focus on the requirement for tests with sensitivity by virtue of the biomarker’s amplification, provided either by PCR or isothermal assays. While it comes to RNA viruses such as SARS-CoV-2, Ebola, or hepatitis, for instance, the “sample preparation” steps also have to navigate around the snags of carrying out a reverse transcription step to produce DNA erstwhile amplification, substantially complicating the process of sample preparation (Witkowska McConnell et al., 2021).
It is important to note that the eminent aspects of applied research require multidisciplinary teams collaborating to address major global challenges comprising antimicrobial resistance, infectious disease, or environmental monitoring and sustainability.[35]
Miniaturization and integration of diagnostic tests are essential elements to consider for the foreseeable evolution of LOCs. Optimizing solidity and consistency without forfeiting sensitivity is a priority in their development, as is establishing novel and reliable interfaces. Multiple parameter measurement in a single test is a noteworthy advancement, as is the development of cell-on-chip or even organ-on-chip platforms that simulate the physiological characteristics of illnesses. Furthermore, the application of nanotechnology can make analyte measurement equipment smaller and more sensitive. Furthermore, LOC devices have the potential to be used in conjunction with next-generation companion diagnostics for personalized (stratified) treatment. The spread of LOC devices outside of a hospital’s central laboratory can minimize expenses and analytical turnaround time.
DISCUSSION
A cross-sectional study was conducted to assess the reliability of the LRP device. According to the aforementioned details of the study, it can conclude that LRP could be a very expedient method for detecting malaria parasites in a clinical laboratory
The alpha variant of SARS-CoV was detected using an LOC device, in a screening test that demonstrated high sensitivity and specificity
According to the series of human experiments performed to determine the total blood cholesterol levels, the precision and celerity of the device have been reiterated and verified
The device has shown noteworthy accuracy in detecting two differently-sized algae using a smartphone-linked microfluidic chip technology
In a screening test conducted to identify various infectious diseases, the device proved to be exact and as efficient as any conventional laboratory diagnosis.
CONCLUSION
Imaging and invasive tissue sampling have become less prevalent in medical diagnostics over time in favor of considerably more minimally invasive techniques that find disease biomarkers in physiological fluids. These biomarkers may be cells, nucleic acids, proteins, or tiny metabolites. Nowadays, the majority of biomarker detection assays are conducted mostly in centralized labs, necessitating trained personnel to operate complicated benchtop analyzers, with an associated prolonged time-to-result interval.[36] In light of the fact that time is frequently of the essence for many medical diseases, the latter factor is crucial. Due to their high expenses and requirement for expert operators, such analyzers are also inherently limited in low-resource contexts.
Due to this, substantial efforts are now being made to create POC tests that may be used at the patient’s site by an amateur.
Such tests should be quick (whose optimum execution duration is between a few seconds and a few hours), accurate, sensitive, specific, and reasonably priced. The optimal scenario for this kind of test would be an autonomous and self-sustaining operation that enables an inept operator to load a sample of extracted body fluid into the device (such as urine, blood, sweat, and saliva,) and procure insightful outcomes with a minimum amount of user participation (i.e., sample in, result out). Comprehensively integrated LOC technologies, which combine all indispensable analytical procedures (including sample loading and preparation in a single device), have the potential to significantly boost the performance of POC testing devices. Despite the fact that LOC applications are still pioneering in its infancy, businesses and applied research teams are becoming increasingly interested in these technologies. These pursuits are not only limited to fields such as cellomics, rapid screening, and pharmaceutics, but also encompass chemical analysis, environmental monitoring, and medical diagnostics.
The device would condense the capabilities of a colossal biochemistry lab into a platform the size of a thumb or even tinier. It is a matter of only a few years, these tiny chips will finally start to emerge from their infancy, poised to make a phenomenal impact in the world.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Commercialization of microfluidic devices. Trend Biotechnol. 2014;32:347-50.
- [CrossRef] [PubMed] [Google Scholar]
- Sensing methods for dielectrophoresis phenomenon: From bulky instruments to lab-on-a-chip. IEEE Circuits Syst Mag. 2004;4:5-15.
- [CrossRef] [Google Scholar]
- Usage of Microfluidic Lab-on-chips in Biomedicine. Proceedings of 12th Biennial Baltic Electronics Conference. In: Tallinn. Vol 5. 2010. p. :249-52.
- [CrossRef] [Google Scholar]
- Simple 3D printed scaffold-removal method for the fabrication of intricate microfluidic devices. Adv Sci (Weinh). 2015;2:1500125.
- [CrossRef] [PubMed] [Google Scholar]
- Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics. Lab Chip. 2013;13:4740-4.
- [CrossRef] [PubMed] [Google Scholar]
- Draw your assay: Fabrication of low-cost paper-based diagnostic and multi-well test zones by drawing on a paper. Talanta. 2015;144:289-93.
- [CrossRef] [PubMed] [Google Scholar]
- Digital dipstick: Miniaturized bacteria detection and digital quantification for the point-of-care. Lab Chip. 2020;20:4349-56.
- [CrossRef] [PubMed] [Google Scholar]
- Microfluidics and BioMEMS Applications Boston: Kluwer Academic Publishers; 2002.
- [CrossRef] [PubMed] [Google Scholar]
- Towards lab-on-a-chip diagnostics for malaria elimination. Lab Chip. 2018;18:75-94.
- [CrossRef] [PubMed] [Google Scholar]
- Microfluidic channel-assisted screening of hematopoietic malignancies. Genes Chromosomes Cancer. 2014;53:255-63.
- [CrossRef] [PubMed] [Google Scholar]
- Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip. 2012;12:3249-66.
- [CrossRef] [PubMed] [Google Scholar]
- Highly sensitive lab on a chip (LOC) immunoassay for early diagnosis of respiratory disease caused by respirable crystalline silica (RCS) Anal Chem. 2019;91:6652-60.
- [CrossRef] [PubMed] [Google Scholar]
- Lab-on-a-chip devices for point-of-care medical diagnostics. Adv Biochem Eng Biotechnol. 2020;179:247-65.
- [CrossRef] [PubMed] [Google Scholar]
- Manufacturing and wetting low-cost microfluidic cell separation devices. Biomicrofluidics. 2013;7:56501.
- [CrossRef] [PubMed] [Google Scholar]
- Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412-8.
- [CrossRef] [PubMed] [Google Scholar]
- Lab-on-a-chip synthesis of inorganic nanomaterials and quantum dots for biomedical applications. Adv Drug Deliv Rev. 2013;65:1470-95.
- [CrossRef] [PubMed] [Google Scholar]
- Sialochemistry: A key to investigation for oral diagnosis. J Indian Acad Oral Med Radiol. 2013;25:121-5.
- [Google Scholar]
- Lab-on-a-chip for oral cancer screening and diagnosis. Head Neck. 2008;30:111-21.
- [CrossRef] [PubMed] [Google Scholar]
- Labon-a-chip technologies for oral-based cancer screening and diagnostics: Capabilities, issues, and prospects. Ann New York Acad Sci. 2007;1098:467-75.
- [CrossRef] [PubMed] [Google Scholar]
- The tooth on-a-chip: A microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip. 2020;20:405-13.
- [CrossRef] [PubMed] [Google Scholar]
- In-vitro models of biocompatibility testing for restorative dental materials: From 2D cultures to organs on-a-chip. Acta Biomater. 2022;150:58-66.
- [CrossRef] [PubMed] [Google Scholar]
- Dentin on the nanoscale: Hierarchical organization, mechanical behavior and bioinspired engineering. Dent Mater. 2017;33:637-49.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro cytotoxicity of dental adhesives: A systematic review. Dent Mater. 2019;35:195-205.
- [CrossRef] [PubMed] [Google Scholar]
- Biocompatibility of biomaterials-lessons learned and considerations for the design of novel materials. Dent Mater. 2017;33:382-93.
- [CrossRef] [PubMed] [Google Scholar]
- Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev. 2010;39:1153-82.
- [CrossRef] [PubMed] [Google Scholar]
- Smartphone based microfluidic lab-on-chip device for real-time detection, counting and sizing of living algae. Measurement. 2022;187:110304.
- [CrossRef] [Google Scholar]
- Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip. 2012;12:2118-34.
- [CrossRef] [PubMed] [Google Scholar]
- COMSOL Assistance for the Determination of Pressure Drops in Complex Microfluidic Channels In: Comsol Conference. 2010. p. :2.
- [Google Scholar]
- Microforming-from basic research to its realization. J Mater Process Technol. 2002;125:35-44.
- [CrossRef] [Google Scholar]
- Recent advances of utilizing artificial intelligence in lab on a chip for diagnosis and treatment. Small. 2022;18:2203169.
- [CrossRef] [PubMed] [Google Scholar]
- Overcoming the limitations of COVID-19 diagnostics with nanostructures, nucleic acid engineering, and additive manufacturing. Curr Opin Solid State Mater Sci. 2022;26:100966.
- [CrossRef] [PubMed] [Google Scholar]
- Lab-on-a-chip platforms for disease detection and diagnosis In: Biosensors and Nanotechnology: Applications in Health Care Diagnostics. Hoboken: Wiley; 2018. p. :155-81.
- [CrossRef] [Google Scholar]
- An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip. 2004;4:310-5.
- [CrossRef] [PubMed] [Google Scholar]
- A polymer lab-on-a-chip for reverse transcription (RT)-PCR based point-of-care clinical diagnostics. Lab Chip. 2008;8:2121-7.
- [CrossRef] [PubMed] [Google Scholar]






