Translate this page into:
Is alveolar osteitis more prevalent since COVID-19? A cross-sectional study

*Corresponding author: M. S. Nishanth, Intern, Department of Oral and Maxillofacial Surgery, NSVK Sri Venkateshwara Dental College and Hospital, Bengaluru, Karnataka, India. nishanthms99@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nishanth MS, Vishwas L, Tantry D. Is alveolar osteitis more prevalent since COVID-19? A cross-sectional study. J Academy Dent Educ 2023;9:5-12.
Abstract
Objectives:
The aim of the study was to analyze the association between post-extraction alveolar osteitis and coronavirus disease 2019 (COVID-19) in a single center.
Material and Methods:
A monocentric cross-sectional study was carried out at NSVK Sri Venkateshwara Dental College and Hospital located in Bannerughatta, Bengaluru in the Department of Oral and Maxillofacial Surgery from January 2021 to August 2022. The study sample comprised 50 participants; an extraction of one or more teeth was performed on women and men between the age group of 15–83 years and returned with complication of alveolar osteitis. Demographic details such as age, gender, medical history, personal habits, COVID-19 immunization history, history of COVID-19, and its complications were acquired from the patient’s case history files throughout the study and recorded. Descriptive statistics were used to analyze the collected data. Chi-square test was used to check for association between the groups.
Results:
Females (62%) were majority of the study participants with an average age of 40 years. Of the patients reported to the hospital, 46% were COVID-positive, 38% were smokers, 48% consumed alcohol, and 80% presented with some underlying systemic condition. A statistically significant association was seen between history of COVID-19 positive patients and systemic condition with P = 0.014 (P ≤ 0.05).
Conclusion:
Patients with systemic conditions were more prone to dry socket; however, personal habits such as smoking, alcohol, and use of tobacco showed no direct relationship. According to study, previous history of COVID-19 infection did not have significant effects in regards to alveolar osteitis. Thus, more research on recovered COVID-19 patients should be done to understand the etiology of dry socket.
Keywords
Alveolar osteitis/dry socket
Coronavirus disease 2019
Extraction
INTRODUCTION
Hundreds of thousands of people were affected by the global pandemic burden brought on by the recently discovered coronavirus disease 2019 (COVID-19), a new, deadly form of the coronavirus, which is spread from person to person. This virus can be fatal not only to people with weak immune systems but even to young, healthy people who are in perfect health.[1]
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is an infectious disease that causes COVID-19 and has presented a serious danger to global health.[2] In December 2019, Wuhan, China, reported the outbreak. It was recognized as a pandemic on March 11, 2020, and a crisis involving public health of international significance on January 30, 2020. By November 6, 2020, 190 nations or regions had reported more than 48.7 million COVID-19 infections, which led to more than 1.23 million fatalities.[3]
This article’s primary goal is to determine whether the incidence of dry socket following routine oral surgical procedures at NSVK Sri Venkateshwara Dental College and Hospital, Bengaluru, in the Department of Oral and Maxillofacial Surgery increased during the COVID-19 pandemic.
The majority of procedures performed by departments of oral surgery are dental extractions. Tooth extractions may be necessary for a variety of reasons, such as pathologies of the pulp or periapex, periodontal disease, caries, pathologies like cysts, or teeth having fracture lines from trauma. Although dental extractions are typically regular and less invasive than other procedures offered in the specialty, problems after surgery can still occur.[4] Dental extraction-related postoperative complications might range from 0% to 35%.[5] Patients will experience several post-operative consequences, which can include hemorrhage, alveolar osteitis, paresthesia of the inferior alveolar nerve, post-operative pain, and infection.[6]
A dry socket, which typically manifests 2–4 days after surgery, is the most common post-operative complication after dental extraction.[7,8] Crawford, in 1876, was the first to elucidate about dry socket.[9] Other names for it include localized osteomyelitis, fibrinolytic alveolitis, septic socket, necrotic socket, alveolar osteitis, alveolalgia, and alveolitis sicca dolorosa.[7]
“Dry socket” colloquially describes a condition seen as a result of the absence of persistent blood clot or a layer of vital, persistent, healing epithelium within the socket and around its occlusal perimeter, and the bone within the socket or around that it is visible after the extraction.[10]
A blood clot could be disturbed by food debris that accumulates inside the extraction socket. The dry socket lesion may take longer to heal if food particles and bacterial biofilm hinder the repairing epithelium from touching the exposed bone. Bacteria can also cause fermentation of food particles inside the socket. This fermentation might produce toxins or antigens that might damage the exposed bone, result in halitosis or an unpleasant taste, and hurt the entire jaw. Evidence, however, indicates that dry socket may not be primarily caused by bacteria.[7]
MATERIAL AND METHODS
Sample design
From January 2021 to August 2022, patients treated in the Department of Oral and Maxillofacial Surgery (OMFS) at Sri Venkateshwara Dental College and Hospital made up the cross-sectional study sample. Baseline information was gathered from databases of patients who had been diagnosed with dry socket and had been documented as part of standard case history recordings. The baseline data also included the COVID vaccination status and COVID history. Fifty patients, all of whom matched the inclusion criteria, were enrolled in the study.
Study design
The demographic information was recorded as part of the customary case history paperwork when they visited the department of OMFS. Before performing the procedure, prophylactic antibiotics and analgesics were administered to symptomatic individuals. The surgical procedures were carried out in sterile conditions. Following the administration of the necessary nerve blocks, all extractions were carried out with the use of local anesthesia containing 2% lidocaine hydrochloride and 1:80,000 adrenaline. Whenever necessary, the surgical wounds were approximated using 3-0 silk sutures. After the surgery, the patients received comprehensive post-operative instructions as well as the appropriate analgesics and antibiotics. Follow-ups were conducted on a regular basis. All the patients who had post-surgical complications were thoroughly evaluated clinically and all the details were recorded in questionnaire format [Figures 1 and 2].

- Questionnaire 1.

- Questionnaire 2.
Inclusion criteria
All of the study’s participants, who ranged in age from 15 to 83 years, had either undergone surgical extractions or extractions of single or multiple teeth and who had returned with dry socket (Alveolar osteitis) with or without COVID-19 history or symptoms. These patients’ follow-up sessions after the extraction surgery were further examined to gather information about their post-operative healing.
Exclusion criteria
Every patient below 15 years of age and those without any post-operative complications, such as dry sockets, and those who did not undergo any extractions were eliminated from the study.
Procedure
Before any procedures were undertaken, patients had their COVID-19 vaccination status checked, and they were asked to bring their COVID-19 test results. Age, gender, systemic medical history, COVID-19 history, site of extraction, type of extraction, and other characteristics including their adverse habit of the patients were recorded as variables in the study. Demographic baseline information and the patients’ medical histories were all gleaned through case history recordings, as previously described. After clinical examination, the patients were instructed to get an intraoral periapical radiograph or orthopantomogram for better assessment. In addition, some individuals had systemic issues such as thyroid disorders, diabetes, hypertension, and asthma. Before the surgery, all of these patients were required to have their doctors’ approval. Patients with diabetes were also counseled to undergo standard check-ups like random and fasting blood sugar tests.
The surgical site was checked for healing when the patients returned for regular follow-ups. Three days after surgery, the patient experienced acute pain that was unmanageable with analgesics and an empty, rancid-smelling socket with loss of the main sutures. Examination of infection and swelling, if present, revealed localized swelling in the surgical site which is tender with discharge happening 3–5 days after surgery. To obtain results for the research, each of these variables was meticulously examined and noted.
After receiving all the data, it was analyzed with the aid of descriptive analysis and inferential statistics using SPSS software. Chi-square test was also used to obtain relationship between the COVID-19 and alveolar osteitis.
RESULTS
The study comprised 50 (n = 50) individuals who underwent tooth extraction and then developed alveolar osteitis between January 2021 and August 2022.
Of the 50 participants, more than half, that is, 27 (54%) did not present with a history of COVID-positive infection [Figure 3].

- COVID history, type of extraction, and systemic conditions.
Among the 50 individuals that participated in the study, females were 31 (62%) and males were 19 (38%) [Figure 4].

- Sex.
Participants in this study ranged in age from 15 to 83 years. Most patients belonged to the age group of 41–50 years (30%), followed by 31–40 years (20%) [Table 1 and Figure 5].
| Age group | Frequency | Percentage |
|---|---|---|
| 15-20 years | 2 | 4 |
| 21-30 years | 12 | 24 |
| 31-40 years | 10 | 20 |
| 41-50 years | 15 | 30 |
| 51-60 years | 5 | 12 |
| >60 years | 6 | 10 |
| Total | 50 | 100 |
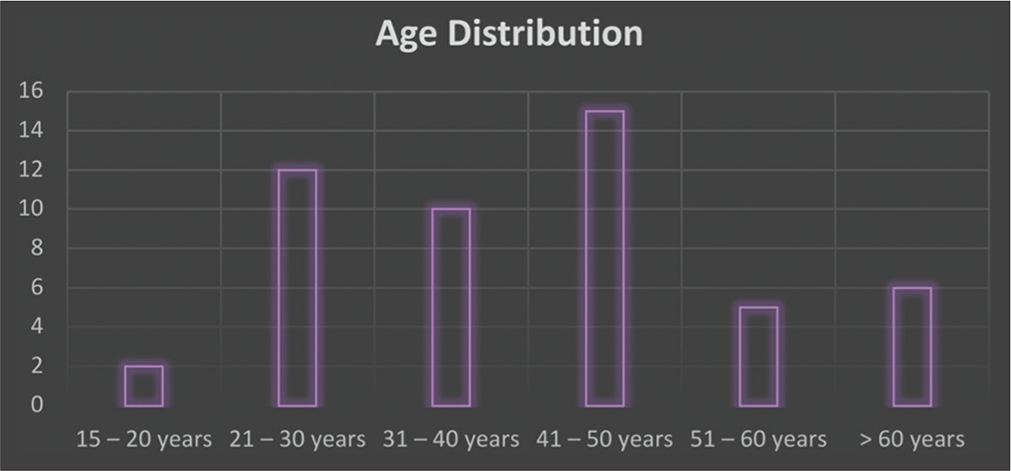
- Age.
Many patients had systemic conditions such as thyroid disorders, diabetes, hypertension, and asthma. Among the participants, 40 (80%) cases had either of the above systemic conditions and 10 (20%) cases did not have any of the above systemic conditions [Figure 3].
A small number of patients came to the department with a history of bad habits, such as smoking, chewing tobacco, paan, gutka, or betel nut, and drunkenness. Among 50 patients who had alveolar osteitis, 19 (38%) patients have a habit of smoking [Table 2], 21 (42%) patients have habit of tobacco use [Table 2], and 23 (46%) patients have habit of alcohol use.
| COVID History | Chisquare* | P-value* | |||
|---|---|---|---|---|---|
| Present n | Absent n | Total | |||
| Smoking | |||||
| Present | 10 | 9 | 19 | 0.543 | 0.563 |
| Absent | 13 | 18 | 31 | ||
| Total | 23 | 27 | 50 | ||
| Tobacco | |||||
| Present | 10 | 11 | 21 | 0.038 | 1.000 |
| Absent | 13 | 16 | 29 | ||
| Total | 23 | 27 | 50 | ||
| Alcohol | |||||
| Present | 8 | 6 | 14 | 0.972 | 0.361 |
| Absent | 15 | 21 | 36 | ||
| Total | 23 | 27 | 50 | ||
Eighteen out of 50 cases of alveolar osteitis turned out to be present in 3rd quadrant, accounting for 36% of all cases, followed by 13 (26%) cases in 2nd quadrant. Eleven (22%) cases of alveolar osteitis were seen in 4th quadrant. Alveolar osteitis was seen only in 8 (16%) patients in 1st quadrant [Figure 6].

- Site of extraction.
Eleven (22%) patients who had alveolar osteitis as a postoperative complication had undergone surgical extraction and 39 (78%) patients had undergone simple/non-surgical extraction [Figure 3].
Majority of the COVID-negative patients with alveolar osteitis that is 36% were non-smokers. No statistically significant relation was observed in patients with alveolar osteitis who had a history of smoking and previous COVID history (P > 0.05) [Table 2].
Most of the COVID-negative patients with alveolar osteitis that is 32% have no habit of chewing tobacco. No statistically significant relation was observed in patients with alveolar osteitis who has the habit of chewing tobacco and previous COVID history (P > 0.05) [Table 2].
Majority of the COVID-negative patients with alveolar osteitis that is 42% have no habit of alcohol use. No statistically significant relation was observed in patients with alveolar osteitis who has the habit of alcohol consumption and previous COVID history (P > 0.05) [Table 2].
Among 50 cases of dry socket, 22 (44%) of cases with either of the above systemic conditions had a positive COVID history. Statistically, there was a significant relationship between patients with alveolar osteitis who had COVID-positive history and has any underlying mentioned systemic conditions P = 0.014 (P ≤ 0.05) [Table 3].
| COVID History | Chisquare* | P-value* | ||||
|---|---|---|---|---|---|---|
| Present n |
Absent n |
Total | ||||
| Systemic conditions | ||||||
| Present | 22 | 18 | 40 | 6.522 | *0.014 | |
| Absent | 1 | 9 | 10 | |||
| Total | 23 | 27 | 50 | |||
Among 50 patients, 20 cases, that is, 40% of alveolar osteitis were seen in non-COVID patients who had undergone simple/non-surgical extraction of the tooth. No statistically significant relation was observed in patients with alveolarosteitis who had previous COVID history and type of extraction (P > 0.05) [Table 4].
| COVID History | Chi square* |
P-value* | |||
|---|---|---|---|---|---|
| Present n |
Absent n |
Total | |||
| Type of extraction | |||||
| Surgical | 4 | 7 | 11 | 0.527 | 0.515 |
| Simple/ | 19 | 20 | 39 | ||
| Non-surgical | |||||
| Total | 23 | 27 | 50 | ||
DISCUSSION
In Wuhan, China, there was an epidemic that gave rise to the coronavirus pandemic. December 2019 through the start of 2020, the coronavirus, which is an infectious disease, spread quickly around the world. It has not only had a negative impact on people’s physical health but also significantly affected their mental health, oral health, and the degree of “health anxiety” they may experience.[11]
COVID-19’s oral manifestation includes – xerostomia, halitosis, parotitis, sialadenitis, and taste disorders such as dysgeusia, hypogeusia, and ageusia. The most frequent oral lesion was an aphthous-like oral ulcer. Herpes-like lesions, candidiasis, glossitis, depapillation, geographic tongue, parotitis, and angular cheilitis were next.[12]
Patients will experience several post-operative consequences, which can include hemorrhage, alveolar osteitis, inferior alveolar nerve paresthesia, post-operative discomfort, and infection. Alveolar osteitis was the most frequent post-surgical consequence.
The purpose of this research was to ascertain the interrelation of the occurrence of alveolar osteitis in patients during the follow-up recall after the surgical procedures in COVID-19 situation.
Alveolar osteitis
According to researchers, the following defines a dry socket: “post-operative pain surrounding the alveolus that increases in severity for some period from 1 to 3 days after extraction, followed by partial or total clot loss in the interior of the alveolus, with or without halitosis.”[7,13]
Alveolar osteitis was initially identified as a side effect of breakdown in the blood clot inside the socket, which manifested 2–4 days following tooth removal.[14] The surrounding mucosa typically turns erythematous, the alveolus empties, and the osseous surrounds are denuded and covered in a layer of yellow-grey necrotic tissue, claim Fazakerlev and Field.[15] Clinically, it is distinguished by a foul smell and severe discomfort that extends to the ear and neck.[16] Pain is the most significant indication of alveolar osteitis. Other symptoms including headache, insomnia, and dizziness may also exist, and its frequency and intensity can vary.[17]
Bone that is exposed and extremely painful to touch may not be protected from mechanical stimulation either by tongue or food particles, leaving the patient in constant, and excruciating pain. All areas of a dry socket lesion, excluding the exposed bone, can be delicately touched with an irrigation needle tip or periodontal probe without causing excruciating discomfort.
Dry socket is generally characterized as “an inflammation in the alveolus of recently extracted teeth, for which discomfort and the time of onset are particular clinical indicators indicative of accurate diagnosis.”
When viewed under a microscope, a dry socket is defined by an inflammatory cellular infiltrate, which includes numerous giant cells and phagocytes in the blood clot, along with the presence of bacteria and necrosis of the lamina dura.[18]
Etiology and pathophysiology of dry socket
Dry socket’s precise cause is not yet known. However, it is known that a number of regional and systemic factors play a role. Dry socket occurs when the clot inside the socket following tooth removal prematurely dissolves, either completely or partially increased local fibrinolytic activity has been identified in clinical and experimental research as a key component in the formation of dry socket.[19,20]
When compared to a normal alveolus, Birn found that the alveolus with a dry socket had more fibrinolytic activity. He emphasized that plasminogen’s direct or indirect activation into the bloodstream produces mediators that cause the partial or whole lyse and disintegration of the clot.[19] After a trauma, the alveolar bone cells release mediators that cause plasminogen to be transformed into plasmin, which ruptures clots by forcing fibrin to disintegrate. This conversion takes place in the presence of additional activators as well as plasmatic or cellular proactivators. The recent classification includes direct (physiologic) and indirect (non-physiologic), as well as subclassifications based on their origins as intrinsic or extrinsic activators.[21]
Intrinsic factors, such as urokinase and factor XII-dependent or Hageman factor, are derived from plasma components. In contrast, direct extrinsic activators, which also include tissue and endothelial plasminogen activators, come from sources other than the plasma. Tissue plasminogen activators are present in the majority of body cells, including the alveolar bone. The bacterial products staphylokinase and streptokinase, which interact with plasminogen to generate an activator complex that transforms plasminogen into plasmin, are among the indirect activators.[21]
Kinins that form in the alveolus are regarded as the root of the common alveolar osteitis pain. The primary bundles of nerve terminations are stimulated by kinins, which may already be responsive to different proinflammatory cytokines and chemicals that produce excruciating pain. In addition, kallikrein is transformed into kinins by plasmin inside the marrow. Hence, the important elements of dry socket could be explained by the presence of plasmin.
Dry socket has also been linked to surgical extractions, particularly those involving flaps and tooth sectioning with an osteotomy level.[22]
Another potential cause of dry socket is the socket’s inclusion of bone and dental remains.[23]
In experimental dry socket models, a variety of bacteria were found in the biologic material of the alveolus, including Strep viridians, Enterobacteria, Bac. coryneform, Proteus vulgaris, Pseudomonas aeruginosa, and Escherichia coli.[24] At infancy, when these microorganisms are typically undetectable in the oral environment, dry socket is a rare occurrence.
Smoking is another trigger for dry socket occurrence.[25] In 1999, Monaco et al. discovered a statistically significant correlation between risky behaviors including smoking and alcohol consumption and difficulties following surgery, such as discomfort and illness.[26] In addition, higher frequency of alveolar osteitis was noted in individuals who were above the age of 18 years. Furthermore, aging was a risk factor, in which other researchers have previously noticed.[27]
Effect of COVID-19 on vascularity
Blood vessel endothelium experiences an inflammatory immunological reaction known as endotheliitis. According to many reports, histological examination and electron microscopy of individuals who died from COVID-19 revealed a build-up of viral inclusions and inflammatory cells.[28]
A systemic inflammatory response must be triggered by SARS-CoV-2’s ability to infect cells of blood vessels and cause a severe inflammatory response locally. Some publications have compared the symptoms of SARS-CoV-2’s systemic inflammation to those of a cytokine storm. This cytokine storm further leads to disseminated intravascular coagulation which has also been detected in COVID-19 infections, but typically in the patients who are seriously unwell.[29]
Commonly encountered coagulation abnormalities in COVID-19 patients point to a hypercoagulable condition known as COVID-associated hemostatic abnormalities or thromboinflammation.[30,31] Elevation of D-dimer is a prevalent finding in patients suffering from COVID-19, especially those who have severe disease.[32] D-dimer forms as a result of the breakdown of fibrin; its presence in the bloodstream indicates that plasmin is breaking down fibrin polymers, and it may be related to the burden of thrombi.
The authors identified hypercoagulability characteristics using whole blood thromboelastography, including a decline in the duration required for fibrin to develop, a reduction in the time it takes for clots to form, and an increase in coagulation strength.[33]
Effect of COVID-19 on bone
By potentially invading the bone cells (osteoblasts and osteoclasts) and interfering with the bone remodeling process, SARS-CoV-2 has the ability to infect the skeletal system. Beside the inflammatory potential, angiotensin converting enzyme 2 (ACE-2)/Angiotensin (1–7) axis has been identified as being linked with osteoporosis, which is indicated by the action of ACE-2 receptors on osteoblasts and osteoclasts.[34]
The vast majority of patients who were older than 35 reported having dry sockets. Several patients arrived at the hospital in great discomfort, and a dry socket diagnosis was made during the inspection. Several individuals disclosed a habit of tobacco smoking as well as hyperglycemia as well as other systemic diseases. Several studies have shown a strong connection between smoking, alveolar osteitis, and diabetes. While there is a general immune system deficit that may have hampered the tissue regeneration, it was found in our study that there was a strong link between individuals suffering from systemic illnesses and COVID-19 history of alveolar osteitis, or diabetes.
As a result, cigarette smoke has chemical irritants that can slow tissue regeneration. Dry socket may be averted by abstaining from smoking, adhering to post-operative guidelines, and practicing good dental hygiene as well as by administering antibiotic therapy to diabetic patients before surgery.
All patients with dry socket were treated by thorough curettage, betadine irrigation followed by betadine ointment pack. All patients received the necessary prescriptions for betadine mouthwash, analgesics, and antibiotics. After 2 weeks of follow-up, the inflammation and infections were under control.
This study’s primary flaw is its extremely tiny sample size, which makes it challenging to draw any relationships or draw any conclusive evidence. The study’s findings are appropriate for this topic, nevertheless due to the study’s small sample size, they cannot be generalized to the total population.
CONCLUSION
Remarkable correlation was seen between patients who had systemic conditions and COVID-positive history with alveolar osteitis. Alveolar osteitis had a significant predilection toward females. All of the cases of alveolar osteitis that was seen had a solid history of smoking or paan chewing, suggesting a substantial link with habit history and the condition. No statistically significant variation was detected in the patients who had COVID history and habit history with alveolar osteitis. However, COVID-19 and alveolar osteitis were not correlated in all the variables such as type of extraction, site of extraction, and habit history, but it was correlated in the patients with systemic conditions. It is interesting to note that every patient who reported of a dry socket also had other systemic conditions such as diabetes, hypertension, and thyroid disorders. Antibiotics and analgesics were administered as preventative measures to each subject in our study. The lower incidence of problems may be explained by the fact that the procedures were carried out under aseptic settings by experienced oral surgeons.
Acknowledgment
We appreciate the help that we received from the Oral and Maxillofacial Surgery team as they guided us through the procedure. We also gratefully appreciate the help with the collection of data and tabulation provided by the juniors and residents in the department of oral and maxillofacial surgery.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- The impact of coronavirus infectious disease 19 (COVID-19) on oral health. Oral Dis. 2021;27(Suppl 3):703-6.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease 2019 (COVID-19): A clinical update. Front Med. 2020;14:126-35.
- [CrossRef] [PubMed] [Google Scholar]
- An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533-4.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of COVID 19 on oral surgery post-operative patient complications and communications within the oral surgery department at the Eastman dental hospital. EurJ Dent Oral Health. 2021;2:8-11.
- [CrossRef] [Google Scholar]
- Infectious postoperative complications in oral surgery. An observational study. J Clin Exp Dent. 2020;12:e65.
- [CrossRef] [PubMed] [Google Scholar]
- Common risk factors for postoperative pain following the extraction of wisdom teeth. J Korean Assoc Oral Maxillofac Surg. 2015;41:59-65.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary views on dry socket (alveolar osteitis): A clinical appraisal of standardization, aetiopathogenesis and management: A critical review. Int J Oral Maxillofac Surg. 2002;31:309-17.
- [CrossRef] [PubMed] [Google Scholar]
- A clinical study of “dry socket”. Int J Oral Surg. 1982;11:226-31.
- [CrossRef] [PubMed] [Google Scholar]
- A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen Psychiatr. 2020;33:e100213.
- [CrossRef] [PubMed] [Google Scholar]
- Oral manifestations in patients with COVID-19: A living systematic review. J Dent Res. 2021;100:141-54.
- [CrossRef] [PubMed] [Google Scholar]
- Update on dry socket: A review of the literature. Med Oral Patol Oral Cir Bucal. 2005;10:77-85.
- [Google Scholar]
- Modern concepts in understanding and management of the “dry socket” syndrome: Comprehensive review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:30-5.
- [CrossRef] [PubMed] [Google Scholar]
- Dry socket: A painful post-extraction complication (a review) Dent Uptade. 1991;18:31-4.
- [Google Scholar]
- A double-blind study on the effectiveness of tetracycline in reducing the incidence of fibrinolytic alveolitis. J Oral Maxillofac Surg. 1989;47:165-7.
- [CrossRef] [PubMed] [Google Scholar]
- Topical treatment of infections of alveolar socket infections following dental extraction. Rev Bras Odontol. 1968;25:82-92.
- [Google Scholar]
- Proteolytic enzyme treatment for the necrotic alveolar socket (dry socket) Oral Surg Oral Med Oral Pathol. 1948;1:608-13.
- [CrossRef] [PubMed] [Google Scholar]
- Etiology and pathogenesis of fibrinolytic alveolitis ("dry socket") Int J Oral Surg. 1973;2:211-63.
- [CrossRef] [Google Scholar]
- Bacteria and fibrinolytic activity in “dry socket”. Acta Odontol Scand. 1970;28:773-83.
- [CrossRef] [Google Scholar]
- Dental extraction wound management: Medicating postextraction sockets. J Oral Maxillofac Surg. 2000;58:531-7.
- [CrossRef] [PubMed] [Google Scholar]
- Alveolar osteitis associated with mandibular third molar extractions. J Am Dent Assoc. 1974;88:802-6.
- [CrossRef] [PubMed] [Google Scholar]
- Medicinal plants in the healing of dry socket in rats: Microbiological and microscopic analysis. Phytomedicine. 2002;9:109-16.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic therapy in impacted third molar surgery. Eur J Oral Sci. 1999;107:437-41.
- [CrossRef] [PubMed] [Google Scholar]
- The extraction of the lower third molars: Germectomy or late avulsion? Minerva Stomatol. 1994;43:191-8.
- [Google Scholar]
- Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1:100052.
- [CrossRef] [PubMed] [Google Scholar]
- Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-7.
- [CrossRef] [PubMed] [Google Scholar]
- Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18:1559-61.
- [CrossRef] [PubMed] [Google Scholar]
- A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4:731-6.
- [CrossRef] [PubMed] [Google Scholar]
- Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231:193-203.e191.
- [CrossRef] [PubMed] [Google Scholar]
- The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas receptor axis as a key player in alveolar bone remodelling. Bone. 2019;128:115041.
- [CrossRef] [PubMed] [Google Scholar]






