Translate this page into:
From the mouth to gut: A microbial journey!
*Corresponding author: S. R. Apoorva, III BDS student, Department of Oral Pathology and Oral Microbiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India. apoodheez10@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Apoorva SR. From the mouth to gut: A microbial journey! J Academy Dent Educ 2020;6(1 & 2):16-22.
Abstract
The oral microbiome invades almost the whole of the body, resulting in “n” number of systemic diseases. The gut is no exception in falling short to them. Many studies both in the four legged animals and their two legged successors (presumed to be the humans) have concluded that the oral microbiome can translocate to the gut and change its microbiota and eventually the immune defense. This ectopic displacement of oral microbiome specifically occurs in severe systemic diseases. Most commonly it is seen having its rage in patients with chronic periodontitis. Dysbiosis in the subgingival microbiota and immune defense, sometimes dysregulation in the gut, turns out to be the threat posed by the oral microbes. Among the other tiny troublemakers, Porphyromonas gingivalis remains the most serious. A dysbiotic gut microbiota may further cause diseases elsewhere in the body. The fact that chronic periodontitis may affect the gut microbiota suggests that the future would foresee a coordinated approach to the treatment of periodontitis and gastrointestinal diseases. Although this specific area of investigation is still a bud, it may portray different pathways for the oral microbiome to cause systemic diseases thence deserving a detailed probe furthermore.
Keywords
Oral microbiome
Virulence factors
Dysbiosis
Gut health
Oral hygiene
INTRODUCTION
A man’s oral cavity hosts more than 700 varieties of microbes.[1] These microbes play an important role in role in modifying human health, thereby leading to disease. The oral microbiota is one of the most complex microbial communities in the whole human body (1). The completion of Human Microbiota Program[2] saw that with the developing trends in the world, people too upgraded their awareness of oral microbes but have not yet analyzed the action of oral microbiota in oral diseases such as caries, periodontal disease, and even few types of oral cancers. Another report from Segata et al.[3] in Genome Biology said that the oral cavity and stool bacteria overlapped in nearly half (45%) of the subjects in the Human Microbiome Project, suggesting that the transfer of oral bacteria to the gut is therefore common. Evidences show that the members of the oral and oropharyngeal microbiota reach the stomach through swallowed saliva, nutrients, and drinks and they are in harmony with digestive diseases. Saliva production ranges from 0.75 to 1.5 L/day but this range depends on the stages of disease progression. The ingested saliva contains an enormous amount of oral microbes. In general, these bacteria are poor colonizers of the healthy intestine.[4] Severe diseases, however, see an increased amount of oral bacteria in the intestine, as in inflammatory bowel disease (IBD), HIV infection, liver cirrhosis, colon cancer, primary sclerosing cholangitis, gastroesophageal reflex disease, and alcoholism.[5] With these microbes as the central target, the treatment of oral and systemic diseases would go hand in hand.This article shall discuss the action of oral microbiota which leads to gut-related diseases.
NATIVES OF THE ORAL CAVITY
One of the most complex environments in the human body is the oral cavity. It constitutes the lips, tongue, teeth, periodontium, hard and soft palate, floor of the mouth, and buccal mucosa, which remains as a gateway between the environment and the body and is the first line of defense against microbes and their products.[6] The oral cavity adds a feather to its cap by serving as a primary digestive organ which helps to breakdown carbohydrates and dietary lipids – the two major energy sources for the host physiology and bacterial growth. This anatomical cavern hosts microbiome of about 700 bacterial species as well as various viruses and fungi. These microbiota can be either aerobic or anaerobic with bacteria being its main inhabitants[3] primarily comprising of the Firmicutes, Bacillus, Proteobacteria, Actinomyces, Fusobacterium, Veillonella, and Streptococcus mutans, Porphyromonas gingivalis, Staphylococcus, and Lactobacillus.[7]S. mutans is the most common visitor and also contributes to the formation of dental plaque[8] and dental caries[9] occurring in hard tissues of the teeth which has the highest incidence among all the other oral diseases. It is followed by P. gingivalis, a periodontal anaerobic bacterium, which when ignored results in the mobility of teeth. Another familiar bacterium is Lactobacillus, a group of microbes that live in the body to benefit the host. Since it is a sugar fermenting bacteria, it can easily cause caries. The next group of microbes in race is around 85 species of fungi, of Candida[10] is the keystone. Candida is dormant in a neutral environment and when the iron is hot, it strikes by forming a biofilm with Streptococcus to play a spoilsport. Few members of the viruses, mainly phages, are also a part.[11] Some of the non-original viruses which appears in the mouth due to certain diseases is the mumps virus and HIV [Table 1]. The oral microbiota can be either beneficial or potentially pathogenic[6] and is kept in balance by the natural defense mechanisms of the oral cavity like the cell shedding from the epithelial surface layers and salivary secretions all of which can limit excessive microbial colonization. Salivary immunoglobulins such as IgA, IgG, and IgM as well as salivary agglutinins, histatins, and lysozymes are a part of the host defense.[12] These oral microbiota are influenced by multiple factors such as personal hygiene, diet, smoke, and sometimes host genetic factors.
| Types of microorganisms involved | Representative members |
|---|---|
| Bacteria | Firmicutes, Bacillus, Proteobacteria, Actinomyces, Fusobacterium, Veillonella,and Streptococcus mutans, Porphyromonas gingivalis, Staphylococcus, and Lactobacillus |
| Fungi | Candida |
| Viruses | Phages, mumps virus, and HIV |
INFLUENCE OF THE CONTRIBUTING FACTORS ON OVERALL HEALTH
Evidences say that oral microbiota has an impact on the systemic health of an individual to an extent. Diet patterns and food extracts influence oral microbiota. In this world of diversity, diet patterns vary from person to person. Some are vegetarians, westerns, and hunter-gatherers.[13] Researchers who have attempted to investigate the impact of oral microbiota using different diet strategies on oral health and physiology found major differences among hunter-gatherers, traditional farmers, western diet, and vegetarians. Through a 16 s short-gun sequencing of salivary DNA, sufficient evidence has shown that abundance ratios of core species are significantly correlated with diet pattern. The galore of Neisseria and Haemophilus showed a huge difference in the hunter-gatherers and westerners and difference in the traditional farmers was in between them. Hunter-gatherers were found to possess few oral pathogens indicating that too much of meat in diet has more tendency to cause oral diseases. The vegetarian diet, on the other hand, alters the oral microbiota’s composition at all taxonomic levels, including the oral pathogens (Neisseria and Haemophilus) and respiratory tract microbes (Campylobacter and Porphyromonas), thereby altering its function. Gene function analysis indicated that the adaptation from hunter-gatherers to western diets may be Vitamin B5 autotrophy and urease-mediated pH regulation.[14] Many studies have shown that intake of green tea or purified catechins helps to prevent oral cancers. When the researchers measured the oral microbiota of tobacco smokers before and after drinking green tea, they found that these smokers were at high risk for oral cancer. The sequencing results showed apparent changes in the relative abundance of Streptococcus and Staphylococcus after green tea intake, which indicated that the tea can change oral microbiota and affect carcinogenesis.[15] Polyphenol is a common food extract found in grapes, cherry, red wine, and apple and its metabolism starts in the oral cavity, but how it influences the oral microbiota still remains a question mark. Of late, alcohol polyphenols were found to exert an antibacterial effect on oral pathogenic bacteria (such as S. mutans), which acts by inhibiting the adhesion of pathogenic bacteria and formation of biofilm. In addition, they can also inhibit the host inflammatory response caused by periodontal pathogens. Thus, polyphenols are considered to be the faith healers against oral pathogens.[16] The tobacco, alcohol, and areca nut users are more prone to oral cancers than the individuals who do not consume them. Researchers have sequenced salivary microbiota to assess the influence of chewing betel nut. Bacterial diversity was found to be considerably reduced among areca nut chewers. Betel nut chewers exhibited an increasing ratio of Actinomyces and Streptococcus and reduced relative abundance of Parascardovia. Thus, oral microbiota may sometimes negatively influence the overall health upon digesting specific food.[17]
PATHWAYS BRIDGING THE ORAL MICROBIOTA AND GUT
Previously, many studies have revealed a mass of orally derived bacteria in the gut of patients with various diseases, but whether these oral bacteria have the potential to induce intestinal inflammation and cause systematic diseases remained unknown,[18] however, recent studies have shown that these orally derived bacteria can colonize the intestines, resulting in the activation of intestinal immune system and chronic inflammation;[19] researchers who ingested saliva samples from patients with Crohn’s disease into germ-free mice found a marked increase in T helper 1 (Th1) IFNalpha+CD4+ cells in the intestinal lamina propria. They also identified a bacterium that is primarily colonized in the colon, named Klebsiella pneumoniae 2H7 (Kp-2H7) which may be the main cause of TH1 cell accumulation. However, its colonization in wild-type mice does not provoke an immune response. All these experiments indicated that this species of bacteria can be colonized in a dysbiotic gut microbiota and act as a pathogen in susceptible hosts. Transplantation of a Klebsiella strain of bacteria isolated from ulcerative colitis into germ-free mice can lead to an immune response and accumulation of TH1 cells. Through the results of 16 s rDNA sequencing, it is found that Klebsiella strain of bacteria in patients with Crohn’s disease increases significantly and in IBD patients, Klebsiella-related genes are found predominant in the gut microbiota.[5] Although many studies have confirmed the close connection between the oral microbiota and digestive diseases, the three possible ways which bridge the oral cavity and digestive system are described as follows:
The oral microbiota tends to directly invade the intestinal tract through the esophagus, creating an imbalance in the intestinal microecology, hence affecting the digestive system [Figure 1][20,21]
According to a study on colorectal cancer, Fusobacterium nucleatum colonizes and shows its action in the colorectal tract by the systemic circulation. Few oral microbes, especially the pathogenic bacterial strain involved in periodontitis, can enter this systemic circulation through the periodontal blood, thereby acting on the whole body [Figure 1][22]
When the human body is in a low-grade inflammatory state (arising due to the metabolites of the oral microbiota in the systemic circulation), there are many opportunities for the occurrence of multiple chronic diseases of the digestive system. This approach is currently not supported by direct evidence from oral microbiological studies. However, there are studies which provide enough sources on systemic disease developing due to an imbalance in gut microbiota which indirectly hints an involvement by the oral microbiota.[23,24]
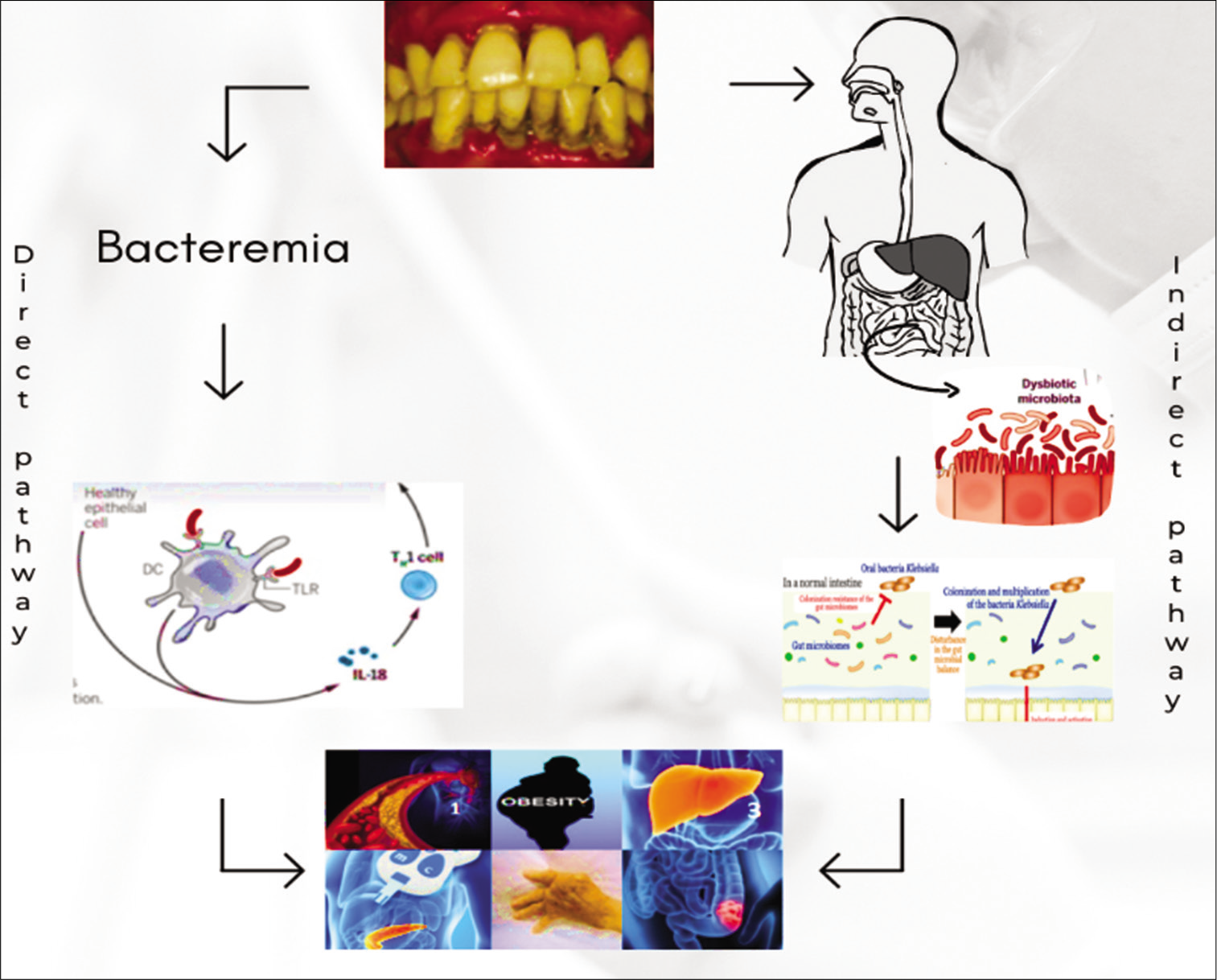
- These are the pathways by which oral microbiota causes gut-related problems. It also shows the systemic complications caused by them.
P. gingivalis is another important bacterium that induces dysbiosis by impairing innate host defenses and disrupting the interaction between host microbiota and mucosa. It can target the complement C5a receptor 1 (C5aR1) and toll-like receptor 2 to activate the PI3K signaling pathway, which blocks phagocytosis and promotes inflammation by secreting SerB, a serine phosphatase, specifically dephosphorylates the p65 NF-B homodimer, which, in turn, inhibits the formation and nuclear translocation of NF-B-p65 homodimers. Transcription of the IL8 gene is reduced, and the IL-8 neutrophil gradient is disrupted during this process. This action will contribute to the cyclical nature of periodontal tissue destruction.[25]
ORAL MICROBIOTA AND THE “ORPHANED” OVERALL HEALTH
The microbes that dwell in the oral cavity remain the mastermind behind wide range of oral diseases, with the caries being predominant followed by periodontal diseases and in some cases, oral cancers. These are also involved either directly or indirectly in causing other metabolic diseases of the gut including diabetes,[26] intestinal bowel disorders,[24] obesity, liver diseases, colon cancers, and pancreatic cancers [Figure 1]. It may also be responsible for an autoimmune disease and rheumatoid arthritis.[27]
Obesity
In today’s world, obesity has become a disease next door. This particular disease has no mercy but it definitely can be prevented. Many reports have described a close relationship between gut microbiota and obesity. According to a study,[26] 33 adult obese people and 29 healthy adults with normal weight were selected to identify the composition of oral microbiota. The study found that oral microbiota in the obese group was quite different from those in the healthy group. However, the bacterial diversity and abundance of oral microbiota in the healthy periodontal obese people were markedly reduced. The oral microbiota of obese people saw a significant rise in the abundance of Plasmodium, S. genus, and S. mutans, whereas there was a fall in the abundance of Haemophilus, Corynebacterium, carbonophilic phage, and Staphylococcus. The environmental adaptability of the oral microbiota of obese people and the biodegradability of exogenous substances were low, and they exhibited notable features of immune diseases. Till date, researchers have only found that there is an imbalance in the oral microbiota of obese people, but the underlying mechanism needs a detailed investigation.[28]
Liver diseases
Previously, it was known that the imbalance of gut microbiota is one of the major factors that lead to the development of liver disease. However, recent studies have reported that imbalance of both gut and the oral microbiota is linked. The diversity and the composition of oral microbiota in patients with liver cancer were significantly different than that of healthy people. Among them, Clostridium, Oribacterium, Ciliate, Actinomyces, and Campylobacter appeared to have high abundance, whereas Haemophilus, Streptococcus, and Pseudomonas have a low abundance. Clostridium and Oribacterium are the potential biomarkers that help in the diagnosis of liver cancer and also distinguish between patients with liver cancer from healthy people. Those with liver cirrhosis too exhibit an imbalance of oral microbiota. In them, there is a reduction in the abundance of oral symbiotic bacteria and an increase in the abundance of potential pathogenic bacteria (e.g., Enterobacteriaceae and Enterococcus). Researchers who compared the gut microbiota of patients with cirrhosis and healthy people found that the intestinal microbiota of patients with cirrhosis is enriched with a variety of oral microbes, including Weirong, Streptococcus, Pasteurella genus, Haemophilus, Lactobacillus, and Clostridium. The researchers revealed that the oral microbes invade gut microbiota of patients with cirrhosis. Studies in animals have shown that P. gingivalis invades the intestinal tract and hence responsible for the changes in gut microbiota’s composition, increased intestinal mucosal permeability and insulin resistance, and results in the spreading of gut’s bacteria to the liver which, in turn, results in an increased triglyceride levels in liver tissue. These changes validated the ability of oral microbes to invade the gut[29,30] in addition to the potentially affecting transplant outcomes, periodontitis is proved to be clinically involved with liver diseases such as pre-cirrhotic non-alcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma;[31] there are chances that the link between the liver and the oral cavity could be made possible through impaired intestinal permeability that, in turn, could allow direct translocation of bacteria along with their products and inflammatory mediators from the oral cavity to the systemic circulation. In addition to periodontitis, cirrhotic patients exhibit numerous other oral issues, such as petechiae, candidiasis, and xerostomia. Although studies regarding a relation between periodontal and liver diseases are not as crystal clear as other studies linking periodontitis and systemic conditions, such as diabetes and cardiovascular disease,[32] a greater incidence of periodontitis (about 25–68%) has been reported in cirrhotic patients compared with healthy controls[33] an observation which definitely requires a detailed probe.
Colon cancer
Reports have shown that along with the gut microbiota, the oral microbiota too is closely related to colorectal cancer. A member of the oral microbiota , F. nucleatum translocates to extraoral sites through systemic circulation and with the decline of immune mechanisms, it leads to local inflammation and indirectly promotes tumor formation. F. nucleatum is a Gram-negative obligate anaerobic bacterium. Several reports have isolated F. nucleatum from colorectal cancer tissues. Patients with high abundance of F. nucleatum have a higher tendency to develop colorectal cancer;[34] hence, the abundance of these bacteria serves as a potential marker for colorectal cancer. Studies reveal that F. nucleatum directly acts on host cells and adheres to normal cells and E-cadherin of cancerous epithelial cells through FadA, hence, activating catenin-regulated transcriptional pathway which leads to increased expression of cancer marker genes which, in turn, promotes cancer. These bacteria can also mediate the entry of non-invasive bacteria (such as Streptococcus and Campylobacter) into cells indirectly promoting the development of tumors. Studies have found that F. nucleatum can also be transferred from the mother’s mouth to fetal tissue and cause fetal death. In patients with colorectal cancer, Han et al. found that the biofilm of their colonic mucosa is consistent with its periodontal biofilm component. However, F. nucleatum was not detected in the stool of patients with colorectal cancer. Hence, it is concluded that F. nucleatum may not translocate from the oral cavity through the digestive tract. The specific mechanism underlying its movement is still unclear, but it may involve transient bacteremia in the bloodstream and then transfer to the colorectal tumor.[35]
Pancreatic cancer
Pancreatic cancer has a high mortality rate and stands fourth among the cancers leading to death. However, its etiology still remains dark. The predominant risk factors that develop pancreatic cancer are genetics, smoking, and obesity. In addition, imbalance of oral microbiota also plays a major role. Helicobacter pylori and P. gingivalis of the oral microbial community are closely related to pancreatic cancer. A study which compared the oral microbiota of 361 patients with pancreatic cancer and 371 patients with non-pancreatic cancer showed that the detection rate of P. gingivalis and Actinobacillus actinomycetemcomitans in the oral cavity of patients with pancreatic cancer appeared to be high. Therefore, the presence of P. gingivalis in the patient’s mouth for a longer period suggests a high risk for pancreatic cancer. It has the ability to promote the occurrence and development of tumors in different ways. In animals, an evasion of the host immune activation both in vivo and in vitro was noticed. P. gingivalis binds to toll-like receptors 2 and 4, activates the NF-B pathway, induces the expression of cytokines (such as TNF, IL-1, IL-6, and IL-8), and forms an inflammation microenvironment, hence promoting tumorigenesis,[36] epidemiological investigations have found a positive relationship between H. pylori and pancreatic cancer. Pancreatic cancers are mostly associated with gastric ulcers. Studies show that the patients with seropositive H. pylori, responsible for gastric ulcers which along with low acid levels and elevated levels of individual nitrosamines, allow the colonization of other bacteria, thereby increasing the risks of pancreatic cancer (i.e., 38%).[37]
CONCLUSION
The impact of oral microbiome on the health of the gastrointestinal system is way beyond our thought process. Although the digestive tract hosts a much greater bacterial density than the oral cavity, the microbes in the latter outnumber the former leading to dysbiosis. Apart from P. gingivalis and A. actinomycetemcomitans, there are a large variety of oral species which can collaborate with the intestinal microbiota through swallowing, regardless of the periodontal status, but only a subset of these microbes seems to colonize the gut especially when the microbiota becomes dysbiotic.[38] These microbes synthesize growth and virulence factors to eradicate the beneficial bacteria in the gut. However, the mechanisms through which they are synthesized both in the oral cavity and the gut remain the same. This inevitably shudders the stability of the commensal microbiota and supports the superiority of orally derived opportunistic pathogens leading to intestinal dysbiosis.[39] Whether this colonization requires only a dysbiotic medium of oral microbiota or an already driven intestinal dysbiosis is not clear, thereby giving a way to further research. However, there are chances for severe diseases and genetic susceptibility of the host to promote ectopic colonization of oral bacteria.[38] When the central target is to treat the oral microbes, oral health can be improved by altering the oral microbiota through a good oral hygiene, periodontal therapy, prebiotics, and probiotics. A report from Takeuchi et al. says xylitol chewing gum has the ability to reduce the load of bacteria in the oral cavity and enhances the oral microecology homeostasis.[40] Further studies on the orally driven intestinal dysbiosis[39] resulting in other systemic inflammatory diseases, colorectal carcinogenesis;[39] changes in the liver[41] will give a better understanding of the effects of oral microbiome on gut health.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The oral microbiota: Dynamic communities and host interactions. Nat Rev Microbiol. 2018;16:745-59.
- [CrossRef] [PubMed] [Google Scholar]
- An intragastric fecal microbiota transplantation program for treatment of recurrent Clostridium difficile in children is efficacious, safe, and inexpensive. J Pediatr. 2018;194:123-7.
- [CrossRef] [PubMed] [Google Scholar]
- Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012;13:R42.
- [CrossRef] [PubMed] [Google Scholar]
- Bacteria from diverse habitats colonize and compete in the mouse gut. Cell. 2014;159:253-2.
- [CrossRef] [PubMed] [Google Scholar]
- Ectopic colonization of oral Bacteria in the intestine drives TH 1 cell induction and inflammation. Science. 2017;358:359-65.
- [CrossRef] [PubMed] [Google Scholar]
- Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21:172-83.
- [CrossRef] [PubMed] [Google Scholar]
- Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113:E791-800.
- [CrossRef] [PubMed] [Google Scholar]
- Newly identified pathogens associated with periodontitis: A systematic review. J Dent Res. 2014;93:846-58.
- [CrossRef] [PubMed] [Google Scholar]
- The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22-38.
- [CrossRef] [PubMed] [Google Scholar]
- Ecology of the oral microbiome: Beyond Bacteria. Trends Microbiol. 2017;25:362-74.
- [CrossRef] [PubMed] [Google Scholar]
- Phage-Bacteria interaction network in human oral microbiome. Environ Microbiol. 2016;18:2143-58.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of host responses and risk for disease progression. Periodontol. 2000-2004;34:57-83.
- [CrossRef] [PubMed] [Google Scholar]
- Oral microbiota: A new view of body health. Food Sci Hum Wellness. 2019;8:8-15.
- [CrossRef] [Google Scholar]
- Oral microbiomes from hunter-gatherers and traditional farmers reveal shifts in commensal balance and pathogen load linked to diet. Mol Ecol. 2018;27:182-95.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of green tea on miRNA and microbiome of oral epithelium. Sci Rep. 2018;8:5873.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of Wine and Food Polyphenols in Oral Health. Vol 69. Netherlands: Trends in Food Science and Technology, Elsevier Ltd.; 2017. p. :118-30.
- [CrossRef] [Google Scholar]
- Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS One. 2017;12:e0172196.
- [CrossRef] [PubMed] [Google Scholar]
- All disease begins in the gut: Influence of gastrointestinal disorders and surgery on oral drug performance. Int J Pharm. 2018;548:408-22.
- [CrossRef] [PubMed] [Google Scholar]
- Talk to your gut: The oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol Rev. 2018;43:1-18.
- [CrossRef] [PubMed] [Google Scholar]
- Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64.
- [CrossRef] [PubMed] [Google Scholar]
- Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One. 2015;10:e0134234.
- [CrossRef] [PubMed] [Google Scholar]
- Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215-25.
- [CrossRef] [PubMed] [Google Scholar]
- The role of the gut microbiome in systemic inflammatory disease. BMJ. 2018;360:j5145.
- [CrossRef] [PubMed] [Google Scholar]
- Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70-89.
- [CrossRef] [PubMed] [Google Scholar]
- The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-kappaB RelA/p65. PLoS Pathog. 2013;9:e1003326.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity alters composition and diversity of the oral microbiota in patients with Type 2 diabetes mellitus independently of glycemic control. PLoS One. 2018;13:e0204724.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontitis and rheumatoid arthritis: What do we know? J Periodontol. 2015;86:1013-9.
- [CrossRef] [PubMed] [Google Scholar]
- Beta-diversity metrics of the upper digestive tract microbiome are associated with body mass index: UGI microbiome beta-diversity associated with BMI. Obesity. 2015;23:862-9.
- [CrossRef] [PubMed] [Google Scholar]
- Deep sequencing reveals microbiota dysbiosis of tongue coat in patients with liver carcinoma. Sci Rep. 2016;6:33142.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260-71.
- [CrossRef] [PubMed] [Google Scholar]
- Interaction between periodontitis and liver diseases. Biomed Rep. 2016;5:267-76.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547-58.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal disease and liver cirrhosis: A systematic review. SAGE Open Med. 2015;3:2050312115601122.
- [CrossRef] [PubMed] [Google Scholar]
- Fusobacterium spp. and colorectal cancer: Cause or consequence? Trends Microbiol. 2013;21:506-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195-206.
- [CrossRef] [PubMed] [Google Scholar]
- Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53.
- [CrossRef] [PubMed] [Google Scholar]
- NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29:42-5.
- [CrossRef] [PubMed] [Google Scholar]
- Can oral Bacteria affect the microbiome of the Gut? J Oral Microbiol. 2019;11:1586422.
- [CrossRef] [PubMed] [Google Scholar]
- Oral Bacteria and intestinal dysbiosis in colorectal cancer. Int J Mol Sci MDPI. 2019;20:4146.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of xylitol-containing chewing gum on the oral microbiota. J Oral Sci. 2018;60:588-94.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2017;2:e94416.
- [CrossRef] [PubMed] [Google Scholar]






