Translate this page into:
Evaluation of biological behavior of odontogenic cysts through epithelial and stromal characterization: A histochemical study

*Corresponding author: Dipanshu Aggarwal, MDS, Senior Lecturer, Department of Oral Pathology and Microbiology, ITS-CDSR, Ghaziabad, Uttar Pradesh, India. dr.dipanshuaggarwal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gupta A, Gupta M, Aggarwal D, Jain A, Shetty DC, Singhal M. Evaluation of biological behavior of odontogenic cysts through epithelial and stromal characterization: A histochemical study. J Academy Dent Educ. 2023;9:59-63. doi: 10.25259/JADE_4_2023
Abstract
Objectives:
Odontogenic lesions include a broad range of pathologies that depend upon an inductive process toward odontogenesis before hamartomatous/neoplastic growth. The epithelium and stroma have an imperative role in pathogenesis and biological behavior. Hence, the study aims to evaluate the biology of the epithelium and stroma in odontogenic cysts (OCs).
Material and Methods:
Histochemical analysis was done (Hematoxylin and Eosin stain, Papanicolaou stain, toluidine blue, and picrosirius red) to determine the presence of keratin, epithelial thickness, mast cells (MCs), and the nature of collagen in OCs.
Results:
Keratin was absent in highly inflamed cases. MCs play a crucial role in cyst pathogenesis. In addition, polarizing microscopy helps in depicting the pattern of collagen fibers.
Conclusion:
The epithelium and the nature of stroma have an imperative role in the biological behavior of OCs.
Keywords
Odontogenic cyst
Dentigerous cyst
Radicular cyst
Picrosirius red stain
Polarizing microscopy
Keratin metaplasia
INTRODUCTION
Odontogenic cysts (OCs) are unique lesions affecting jaws. The origin of these cysts could be inflammatory or developmental in relationship with the epithelium of the odontogenic apparatus. Radicular cysts (RCs) are the most common cysts followed by dentigerous cysts (DCs), and odontogenic keratocysts (OKCs).[1] These cysts have an overlapping clinico-radiologic appearance.
Under the influence of inflammation and other environmental factors, metaplastic changes, such as keratinization, mucous cells, and ciliated cells, can occur in the odontogenic epithelium as adaptive changes.[2-4] Breakdown of the stromal matrix leads to increased osmotic pressure in cystic fluid, which leads to bone resorption; these lesions can damage bone and cause expansile growth in jaws.
The etiopathogenesis of development and growth is yet unknown. It is recognized, however, that a variety of inflammatory cells has a role in this process. Mast cells (MCs) have round, circular to elliptical morphology with hyperchromatic nuclei and basophilic cytoplasmic secretory granules rich in proteoglycans, proteases, and other compounds. MCs have a role in inflammation and bone resorption, as well as interacting with other immune system cells.[5]
Histamine, tryptase, and kinases are among the active components found in MC granules, all of which have proinflammatory or inhibitory effects. Furthermore, heparin might act as both stimulators and inhibitors of the microenvironment signaling pathway. Other mediators generated by stromal cells such as fibroblasts as well as endothelial cells proximal to MCs, such as cyclooxygenase byproducts and heparins, may impact the pathophysiology of these lesions.[6]
Collagen is a crucial component of connective tissue stroma, as it helps to preserve structural integrity and determine tissue function. Natural birefringence in collagen is due to the organization of fibers, amplified by stains such as picrosirius red (PSR), that may be used to distinguish between procollagens, intermediate collagen fibers, and pathological collagen fibers.[7]
Although some studies have demonstrated that extracellular matrices have been involved in the etiopathogenesis of OCs and tumors. However the function of collagen in these pathologies is yet unknown. Therefore, the goal of the research is to learn more about the pathogenesis of OCs by examining and comparing the epithelium and connective tissue stroma characterization using histochemical stains in light microscopy and polarizing microscopy.
MATERIAL AND METHODS
The analysis was performed on 30 tissue specimens retrieved from the archives of the department, which included 10 cases of each OKCs, RCs, and DCs was taken and grouped as Group I, II, and III, respectively. Four paraffin-embedded sections of thickness 4 mm were prepared from each of the 30 cysts. Among those four sections, one was stained with Hematoxylin and Eosin (H and E) for evaluating epithelial hyperplasia, one with Papanicolaou (PAP) stain for observing keratinization and keratin metaplasia, one with PSR stain in combination with a polarizing microscope to analyze for collagen fibers and one with toluidine blue to detect and quantify MCs density [Table 1]. The age groups were subcategorized into two groups, 18–35 years and 35–50 years. Furthermore, histopathological findings and demographics of patients were recorded. The analysis was carried out under different magnifications by two observers to eliminate bias. The data were entered in an Excel spreadsheet and statistical analysis using SPSS v20 was performed.
| Stain | Inference |
|---|---|
| H and E stain | Routine histopathology |
| PAP stain | Keratin metaplasia |
| Toluidine blue | Mast cells evaluation |
| PSR stain | Collagen fibers |
| PSR stain under polarizing microscope | To determine the nature of collagen |
PSR: Picrosirius red, PAP: Papanicolaou
RESULTS
The clinicopathological profile of the 30 cases of OC was studied using different stains. H and E was used as a routine stain, PAP stain was used to study keratin metaplasia, toluidine blue for MC evaluation, and PSR stain in conjunction with a polarizing microscope to study the collagen fibers and identify their nature. Out of 30 patients, 16 were females and 14 were males. The age groups were subcategorized into two groups, 18–35 years and 35–50 years, which included 12 and 18 patients, respectively [Table 2].
| Demographic data | |
|---|---|
| Age (years) | |
| 18–35 | 12 |
| 35–50 | 18 |
| Gender | |
| Male | 14 |
| Female | 16 |
| Groups | |
| Group I odontogenic keratocyst | 10 |
| Group II dentigerous cyst | 10 |
| Group III radicular cyst | 10 |
Epithelial hyperplasia was absent in 70% of cases of DC (Group II) compared to OKC (Group I) where it was observed in 80% of cases. The results were statistically insignificant [Figure 1].
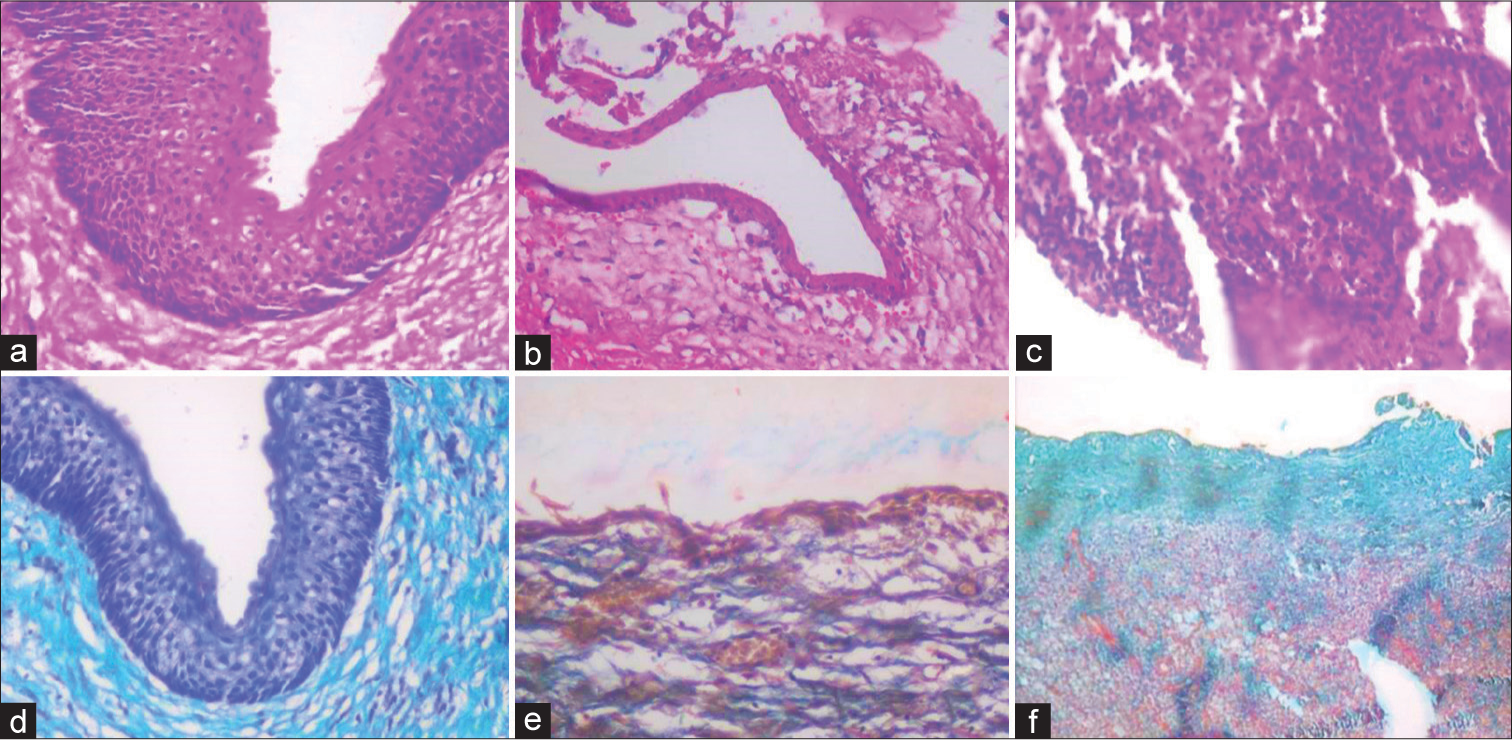
- (a) H and E stained photomicrograph of odontogenic keratocyst (OKC) at ×40 magnification, (b) H and E stained photomicrograph of dentigerous cyst (DC) at ×10 magnification, (c) H and E stained photomicrograph of a radicular cyst at ×10 magnification, (d) Papanicolaou (PAP) stained photomicrograph of OKC at ×40 magnification, (e) PAP stained photomicrograph of DC at ×10 magnification, (f) PAP stained photomicrograph of radicular cyst at ×10 magnification.
Keratin metaplasia was absent in 70% of cases of Group II (DC) compared to a high degree of metaplasia of keratin in 50% of cases of Group III (RC). The results were statistically significant.
MC count was absent in 80% of cases in Group II (DC), compared to low MC count in 30% of cases of Group I (OKC), and high MC count in 70% of cases of Group III (RC). The results were statistically significant.
Collagen fibers observed using PSR stain revealed that 70% of cases in Group I (OKC) showed immature collagen, compared to mature collagen fibers in 60% of cases of Group II (DC), and mixed collagen was observed in 20% of cases of Group II (DC) and III (RC) and 10% cases of Group I (OKC). The results were statistically insignificant [Table 3 and Figure 2].
| Parameters | Group I (OKC) | Group II (DC) | Group III (RC) | P-value |
|---|---|---|---|---|
| n(%) | n(%) | n(%) | ||
| Epithelial hyperplasia | ||||
| Absent | 2 (20) | 7 (70) | 4 (40) | 0.56 |
| Present | 8 (80) | 3 (30) | 6 (60) | |
| Keratin metaplasia | ||||
| Absent | 2 (20) | 7 (70) | 2 (20) | 0.02 |
| Low | 5 (50) | 2 (20) | 3 (30) | |
| High | 3 (30) | 1 (10) | 5 (50) | |
| Mast cells | ||||
| Absent | 4 (40) | 8 (80) | 0 | 0.01 |
| Low | 3 (30) | 1 (10) | 1 (10) | |
| Moderate | 2 (20) | 1 (10) | 2 (20) | |
| Dense | 1 (10) | 0 | 7 (70) | |
| Collagen fibers | ||||
| Immature | 7 (70) | 2 (20) | 4 (40) | 0.07 |
| Mature | 2 (20) | 6 (60) | 4 (40) | |
| Mixed | 1 (10) | 2 (20) | 2 (20) |
OKC: Odontogenic keratocyst, DC: Dentigerous cyst, RC: Radicular cyst
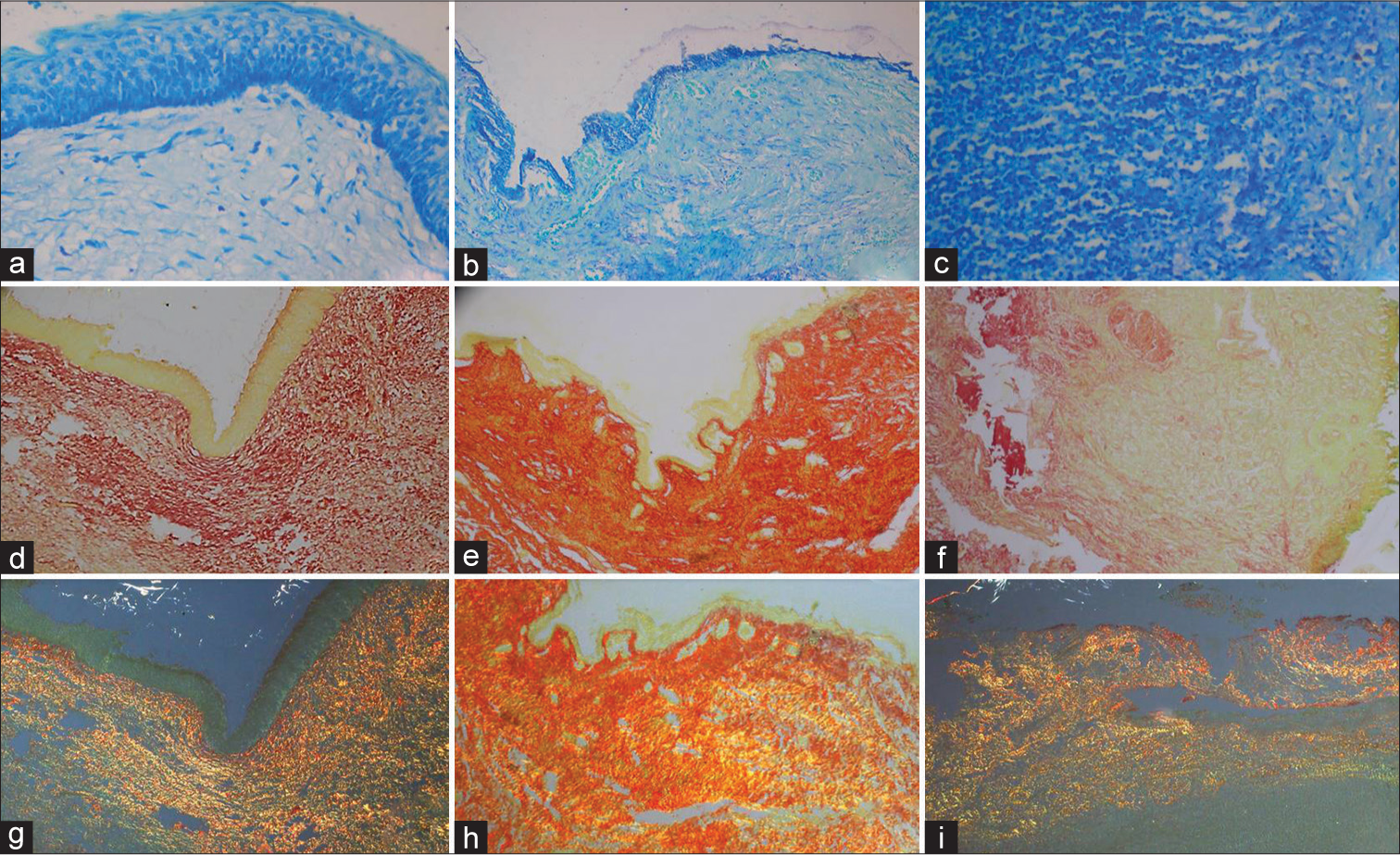
- (a) Toluidine blue stained photomicrograph of odontogenic keratocyst (OKC) showing mast cells (MCs) at ×40 magnification, (b) Toluidine blue stained photomicrograph of dentigerous cyst (DC) showing MCs at ×10 magnification, (c) Toluidine blue stained photomicrograph of radicular cyst showing MCs at ×40 magnification, (d) Picrosirius red (PSR) stained photomicrograph of OKC showing collagen at ×40 magnification, (e) PSR stained photomicrograph of DC showing collagen at ×10 magnification, (f) PSR stained photomicrograph of radicular cyst showing collagen at ×40 magnification, (g) PSR stained photomicrograph of OKC showing immature collagen bundles under the polarized microscope at ×40 magnification, (h) PSR stained photomicrograph of DC showing mature and immature collagen bundles under the polarized microscope at ×10 magnification, (i) PSR stained photomicrograph of Radicular cyst showing mature collagen bundles under the polarized microscope at ×10 magnification.
DISCUSSION
Epithelial-mesenchymal interactions (EMI) influence morphogenesis and cytodifferentiation during odontogenesis.
In addition, these interactions lead to the development of OCs and tumors. The majority of the research has focused on assessing the proliferative capacity of the epithelium of OCs with limited attention to the correlation between this epithelium and collagen interaction.[8]
Keratin in the cystic lining of DCs and RCs is referred to as keratin metaplasia. In the present study, 50% of cases of RCs exhibited keratin metaplasia, compared to 70% of DC cases where no keratin was seen. This difference is because individuals with mild and moderate inflammation had more keratinization, whereas those with severe inflammation had none at all. Our results contrast with those of Brown, where keratin was observed in 2.5% of DC cases and 2% of dental cyst cases.[2]
Moreover, we also observed that mild–moderate inflammation could lead to hyperplastic cystic lining and active keratin production in 60% of cases showing keratin metaplasia in Group III with epithelial hyperplasia. The use of PAP staining, which is specific for keratin, is responsible for the observed keratin variation.
Similarly, Maheswaran et al. examined keratin metaplasia with the clinical presentation of OCs and found that out of 71 cases of various OCs, 36.6% displayed keratinization. Keratin was also found in residual cysts in individuals over 45 years of age, along with epithelial hyperplasia. However, the results were statistically insignificant, leading to the inference that keratin metaplasia may be regulated by inflammation.[9]
MCs can be activated to produce such a variety of mediators, in so many different settings, that it has been tempting to suggest that they are involved in the pathogenesis of many diseases. The present study showed a high MC count in 70% of RC cases. Our results are in harmony with the study done by Pinheiro et al., where they inferred that increased MCs were seen in small-sized RC, which relates to increasing permeability. They concluded that MCs have a role in the initiation of RCs, whereas in OKCs, MCs contribute to bone resorption and expansion of the lesion.[10]
Similarly, Farhadi et al. elucidated the activity of MCs in the pathogenicity of OCs and discovered a higher density of MCs in keratocystic odontogenic tumors when compared to DCs, inferring that MC activity was highest in the outermost capsule of the cyst, which is in proximity to bone. MCs modulate osteoclastic activity, leading to greater bone resorption through the release of cytokines like heparin.[11]
On the contrary, Rajabi-Moghaddam et al. found no significant difference in MC count between OKCs, DCs, and RCs and suggested that high activity in the capsule of OCs is correlated with their growth. MCs contribute to increased osmotic pressure through various mechanisms, including heparin release in cystic fluid, enzymes that degrade stroma, histamine, and increased vascular permeability resulting in transduction of serum proteins. Moreover, the production of prostaglandins by MCs is related to bone resorption.[12]
Fonseca-Silva et al. analyzed MC and vascular endothelial growth factor (VEGF) expression as well as microvessel density (MVD) between periapical cysts and granulomas and discovered that cysts had the most MCs in comparison. Furthermore, the presence of VEGF and MVD was in line with the immunological processes taking place in the lesions. The authors interpreted that the existence of MCs is linked to vasodilation, proteoglycan creation, angiogenic response, collagen synthesis, inflammatory modulation, bone resorption, and increasing cystic fluid as the damage progresses.[13]
The nature of collagen fibers plays a pivotal role in predicting the biological behavior of odontogenic lesions. In the present study, stromal collagen fibers were studied using PSR special staining, which showed that 70% of OKCs revealed immature collagen. Further studies have shown similar results; Jahagirdar et al. compared collagen fibers of OCs and OTs by PSR special stain and observed yellowish-orange birefringence in developmental cysts, suggesting well-oriented collagen, which relates to a poor prognosis. This finding is comparable to stromal reactions of odontogenic tumors and may explain the clinicopathological aggressive behavior of these cysts.[14]
Furthermore, Aggarwal and Saxena compared the pattern of collagen fibers in OCs and tumors and concluded that analogous birefringence among OCs and OTs could imply a common histogenesis of these lesions. Different RC patterns reflect different biological activity and a favorable function for inflammation in collagen fiber polarization.[15]
Hirshberg et al. explored the inflammatory consequences in OKCs using PSR and concluded that inflammation affects collagen orientation in the wall of OKCs. This effect is evidenced by their birefringence colors under polarized light. The fraction of thick fibers with green birefringence is reduced by intense inflammatory reactions, whereas thick fibers showing red birefringence rise and seem more packed.[16] Vij et al. investigated the collagen type in the capsule of OCs and determined if the nature of the overlying epithelium may be affected by the capsular connective tissue, stressing the phenomenon of EMI in cyst biological behavior. The authors concluded that inflammation can cause changes in collagen and that polarizing dye and microscopy techniques are valuable aids in investigating collagen.[7]
CONCLUSION
The present study aimed to evaluate the biological behavior of OCs through epithelial and stromal characterization using histochemical techniques. The findings shed light on the significance of EMI in odontogenesis, morphogenesis, and cytodifferentiation during the development of OCs and tumors. The study revealed intriguing contrasting results regarding keratin metaplasia, MC presence, and collagen fiber characteristics among different types of OCs. Overall, the study provided valuable insights into the biological behavior of OCs, emphasizing the importance of EMI in their pathogenesis. The contrasting findings underscored the need for further research and comprehensive investigations to elucidate the underlying mechanisms governing the behavior of OCs. These results may contribute to the advancement of knowledge in the field of OC research and potentially influence future diagnostic and therapeutic approaches for these intriguing lesions.
Ethical approval
The author(s) declare that they have taken the ethical approval and the ethical approval number is ITSCDSR// IIEC/RP/2022/008.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Odontogenic Cysts. Dental Clinics of North America. 2020;64(1):105-119.
- [CrossRef] [PubMed] [Google Scholar]
- Metaplasia and degeneration in odontogenic cysts in man. J Oral Pathol. 1972;1:145-58.
- [CrossRef] [PubMed] [Google Scholar]
- Age changes in residual radicular cysts. J Oral Pathol. 1986;15:524-8.
- [CrossRef] [PubMed] [Google Scholar]
- Mucous and ciliated cell metaplasia in epithelial linings of odontogenic inflammatory and developmental cysts. J Oral Sci. 2005;47:77-81.
- [CrossRef] [PubMed] [Google Scholar]
- Mast cells in periapical lesions: potential role in their pathogenesis. J Oral Pathol Med. 2010;39:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Degranulating mast cells in fibrotic regions of human tumors and evidence that mast cell heparin interferes with the growth of tumor cells through a mechanism involving fibroblasts. BMC Cancer. 2005;5:121.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of collagen in connective tissue walls of odontogenic cysts-A histochemical study. J Oral Pathol Med. 2011;40:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical analysis of patterns of p53 and PCNA expression in odontogenic cystic lesions. Med Oral Pathol Oral Cir Buccal. 2008;13:E275-80.
- [Google Scholar]
- Keratin metaplasia in the epithelial lining of odontogenic cysts. J Pharm Bioallied Sci. 2014;6(Suppl 1):S110-2.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between mast cells and E-cadherin in odontogenic keratocysts and radicular cysts. Clin Oral Investig. 2020;24(1):181-191.
- [CrossRef] [PubMed] [Google Scholar]
- The Possible Role of Mast Cells in the Odontogenic Cyst's Pathogenesis: A Comparative Study between Dentigerous Cyst and Keratocystic Odontogenic Tumor. Patholog Res Int. 2016;2016:8754567.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of Mast Cells Count in Odontogenic Cysts Using Histochemical Staining. Iran J Pathol. 2015;10(2):105-10.
- [Google Scholar]
- Detection and quantification of mast cell, vascular endothelial growth factor, and microvessel density in human inflammatory periapical cysts and granulomas. Int Endod J. 2012;45(9):859-64.
- [CrossRef] [PubMed] [Google Scholar]
- Stromal characterization and comparison of odontogenic cysts and odontogenic tumors using picrosirius red stain and polarizing microscopy: A retrospective and histochemical study. Indian J Cancer. 2015;52(3):408-12.
- [CrossRef] [PubMed] [Google Scholar]
- Stromal differences in odontogenic cysts of a common histopathogenesis but with different biological behavior: a study with picrosirius red and polarizing microscopy. Indian J Cancer. 2011;48(2):211-5.
- [CrossRef] [PubMed] [Google Scholar]
- Collagen fibres in the wall of odontogenic keratocysts: a study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999;28(9):410-2.
- [CrossRef] [PubMed] [Google Scholar]






