Translate this page into:
Black fungus – An emerging threat

*Corresponding author: Karthik Rajaram Mohan, Associate Professor, Department of Oral Medicine and Radiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India. drkarthik@vmsdc.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Kumar N, Rajaram Mohan K, Venkatesha R, Fenn S. Black fungus – An emerging threat. J Academy Dent Educ. 2024;10:25-32. doi: 10.25259/JADE_1_2023
Abstract
The term “black fungus” is referred to fulminating, dreadful fungus mucormycosis infection, so-called because of the black-colored necrotic areas it causes due to the black-colored colonies of the fungus, which thrives in infected tissues. Recently, in this COVID-19 pandemic, it has emerged as a new threat to the world. This article enlightens us with the predisposing factors, pathogenesis, types, clinical features, diagnostic modalities, and treatment of Mucormycosis.
Keywords
Black fungus
Mucormycosis
Osteomyelitis
Maxilla
Rhinorbital
INTRODUCTION
The increased incidence of air pollution was one of the etiological factors in the number of emerging cases of mucormycosis. Like Candida albicans, mucormycosis is also one of the normal inhabitants of the oral cavity. When favorable condition exists, mucormycosis virulence increase and cause destructive effects in the affected host.[1] Recently mucormycosis is more commonly encountered among people of India due to the COVID-19 pandemic. Mucormycosis is a dreadful fungus that belongs to the class “Zygomycetes,” subphylum “Mucormycotina,” order “Mucorales.”[1]
The first case of mucormycosis was described in 1885 by Friedrich Kuchenmeister, who died of cancer and an autopsy of his lung revealed numerous hemorrhagic infarcts.[1] The first case of mucormycosis was reported in a 15-year-old boy in Ahmedabad, Gujarat. Cases of mucormycosis were also reported during the natural disaster Tsunami during 2004 that occurred in the Indian Ocean, and a cutaneous form of mucormycosis was reported in Missouri tornado during 2011.[2]
ORIGIN OF MUCORMYCOSIS
Mucormycosis is present all over the environment, in the air, and more commonly in the soil, animal dung, and compost piles.[3] These saprophytic fungi are called Mucormycetes and belong to the family Mucoraceae, class Phycomycetes, scientific order “Mucorales” belong to the genus Mucor and Rhizopus. The most common types of fungi that cause mucormycosis include Rhizomucor species, Cunninghamella bertholletiae, Lichtheimia, Saksenaea, Apophysomyces, Rhizomucor, and Syncephalastrum species.[4,5] Mucor indicus, a species of mucormycosis, was found to affect a child with the left ventricular assist device.[6]
ULTRASTRUCTURAL VIEW OF MUCORMYCOSIS
Comorbidities such as uncontrolled diabetes mellitus with diabetic ketoacidosis, HIV/AIDS, post-COVID patients (recovered from COVID-19 infection), hematological malignancy like acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, chronic lymphocytic leukemia, multiple myeloma, lymphoma, myelodysplastic syndrome, solid organ transplantation – after kidney, liver, heart, lung transplantation, cancer chemotherapy for malignancies, alcoholic liver cirrhosis, hepatitis B or C infection, autoimmune hepatitis, cryptogenic cirrhosis, injudicious use of i.v. desferrioxamine therapy, B-complex with zinc supplements, steroids, antifungals, non-sterile linens, adhesive bandages, pre-packaged food, post-stem cell transplantation, insertion of catheter tubes, insulin pumps, dialysis catheters, defective ventilation system, tooth extraction, serum iron overload due to multiple transfusions or patients undergoing dialysis, intravenous drug users (IVDU) such as cocaine, heroin, and methamphetamine were also a threat for mucormycosis.[6-7]
The stages of mucormycosis described in Figure 1.
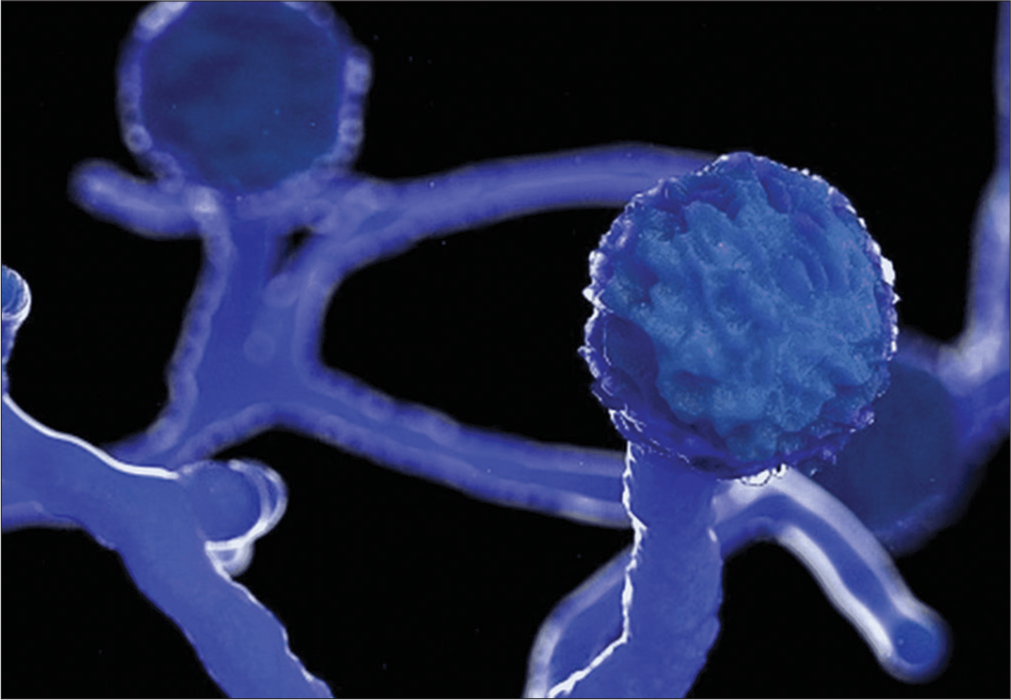
- Ultrastructural view of Mucormycosis-Image courtesy: Mucormycosis: The “black fungus” maiming COVID patients in India-BBC News. Available from https://www.bbc.com/news/world-asia-india-57027829.
Pathogenesis of mucormycosis
Fungi enter the skin through cuts, scratches, burns, or any type of trauma, such as road traffic accidents or through inhalation of decayed fruit and vegetables by autoinoculation. High glucose levels, low oxygen concentrations, high iron levels, and an acidic environment all contribute to the germination of bacteria in the nasal cavity. The spores of mucormycosis invade the nasal mucosa, and the first line of defense cells, polymorphonuclear neutrophils, fail to phagocytize the spores due to impaired glutathione pathway and excess iron in serum. The fungus thrives in an acidic environment, and neutrophil response is absent in patients with diabetic ketoacidosis since the ketoreductase enzyme is used by the fungus in diabetic ketoacidosis. The moist nasal mucosal lining favors the growth and germination of Mucorales fungus, followed by an invasion of fine capillary blood vessels occur hyphae and subsequent thrombosis of the fine vasculature ensues, resulting in infarction of the involved nasal tissue and subsequently, the appearance of dry gangrene with increased mucosal thickening or opacification and either partial or complete obliteration of nasal or maxillary sinus cavity. Such devitalized mucosa is revealed only on contrast-enhanced magnetic resonance imaging (MRI) imaging, as contiguous non-enhanced foci of tissue within the nasal cavity or maxillary sinus. Abnormal communication between the oral cavity and maxillary sinus or nasal cavity occurs as a result of oroantral fistula caused by fungal-induced osteomyelitis and destruction of the underlying bone, either alveolus or hard palate or floor of the nasal or maxillary sinus. Fungal hyphae produce rhizoferrin, which binds iron avidly, and the iron-rhizoferrin complex then binds to fungi for intracellular functions. Corticosteroids elevate blood glucose levels and also prevent the migration of macrophages toward invading fungi and also the fusion and engulfment of mucor fungi by the formation of phagolysosomes.[6,7] The intraoral clinical features and cone beam computed tomography (CBCT) sections of maxilla affected by mucormycosis were shown in Figure 2.
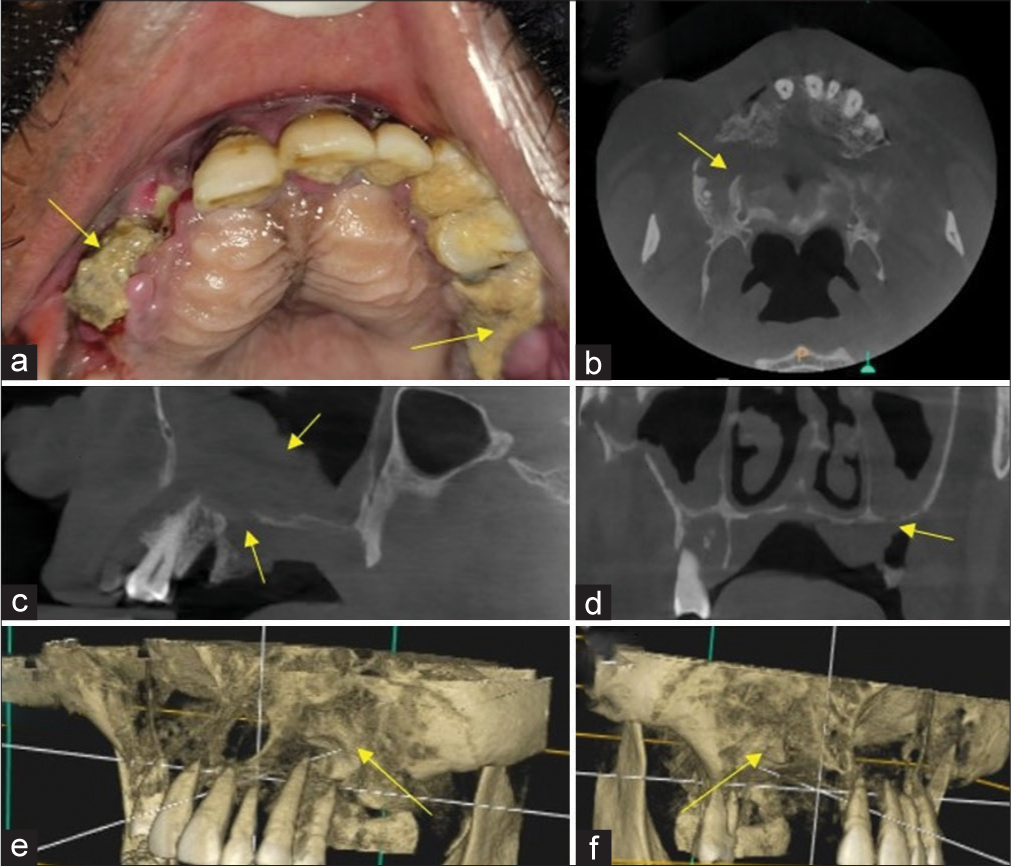
- Yellow arrow in (a) Mucormycosis induced osteomyelitis of maxilla showing exposure of underlying necrotic alveolar bone, Yellow arrow in (b) Axial section cone beam computed tomography (CBCT) showing destruction of maxillary alveolar bone, Yellow arrow in (c) Sagittal section CBCT showing mucosal thickening within maxillary sinus and destruction of floor of maxillary sinus, yellow arrow in (d) Coronal CBCT sections revealed destruction of floor of maxillary sinus, yellow arrow in (e and f) 3D reconstructed CBCT images of Maxilla showing destruction of maxillary alveolar bone. Blue lines in (e and f) indicates orthogonal planes.
High doses of corticosteroids greater than three weeks favor the formation of mucormycosis. Invasion occurs into the maxillary sinus through the sphenopalatine fossa, from where it spreads to the orbital cavity through the ethmoid sinus. Pterygopalatine fossa helps to disseminate infection to other locations. Hyperglycemia results in glycosylation of iron-transporting proteins transferrin and ferritin, decreases their iron binding capacity, in addition, diabetic ketoacidosis results in accumulation of ketone bodies (β-hydroxy butyrate), which lowers the pH in blood vessels, that impairs the ability of transferrin to chelate iron leading to increase in iron, glucose, and β-hydroxybutyrate, thus enhancing the growth of fungus.[6,7]
The incidence of iron overload is high in dialysis and multiple transfusion patients. The Mucorales use an oral iron chelator called desferrioxamine as a xenosiderophore to acquire iron from the host, which increases the risk of mucormycosis in such patients.[6,7]
Duffy et al. stated that hospital linens favored transmission of mucormycosis, and hence they must be frequently laundered and stored in packages that minimize their exposure to environmental contaminants.[8]
TYPES OF MUCORMYCOSIS
Rhino cerebral mucormycosis, Rhino-orbitocereral mucormycosis mucormycosis, pulmonary mucormycosis, cardiac mucormycosis, gastrointestinal mucormycosis, renal mucormycosis, necrotizing cutaneous mucormycosis, and disseminated mucormycosis.
Rhinocerebral mucormycosis
IVDUs such as cocaine, methamphetamine, and heroin are the people at risk for developing rhinocerebral mucormycosis. The most common clinical features include foul smelling rhinorrhea, nasal congestion, bleeding from the nose, reduced visual acuity, non-healing ulcer involving supporting alveolus and hard palate, tooth mobility, and multiple discrete intraoral swelling involving attached gingiva resembling multiple periodontal or periapical abscess. Patients presenting clinically with multiple discrete swellings involving gingiva alveolus must also be screened for random and glycated hemoglobin levels to rule out uncontrolled diabetes. Cerebral abscess can develop, leading to skull base erosions with cranial palsies.[9-11]
Mane et al. reported the occurrence of facial palsy as a clinical presentation with rhinocerebral mucormycosis and can be misdiagnosed as cerebrovascular accident (stroke) in such affected patients.[12]
Cutaneous mucormycosis
Invasive cutaneous mucormycosis can present clinically as vesiculobullous lesion.[13]
Peritoneal mucormycosis
It occurs in ambulatory patients undergoing peritoneal dialysis.[14]
Acute subdural hematoma and intracerebral hemorrhage can occur as a rare complication of rhinocerebral mucormycosis.[15]
Gastrointestinal mucormycosis
Rhizopus species encountered in gastrointestinal mucormycosis. Ingestion of contaminated food, tablets, and tongue depressors are predisposing factors for gastrointestinal mucormycosis. Gastric perforation resulting in gastrointestinal fistula occur as a complication of gastrointestinal mucormycosis.[16,17]
Splenic mucormycosis
It can occur in patients with aplastic anemia.[18]
Necrotizing cutaneous mucormycosis
Mucor irregularis was found by the etiological factor for the primary cutaneous mucormycosis. Contamination by non-sterile bandages, adhesives, and linen was found to be the etiological factors. The invasive cutaneous form of mucormycosis can mimic as a vesiculobullous lesion in a neonate.[19] The skin lesions due to cutaneous mucormycosis can mimic pancreatic or gouty panniculitis.[20]
Oral mucormycosis
It can occur as a result of allogeneic stem cell transplantation done as a treatment for aplastic anemia. The clinical features of oral mucormycosis are palatal ulceration with exposure of underlying necrotic bone, tooth mobility, halitosis, and facial pain.[21,22]
Pulmonary mucormycosis
Cunninghamella species were frequently encountered in patients with pulmonary mucormycosis. Mostly encountered in patients with neutropenia. Patients affected by pulmonary mucormycosis have difficulty in breathing, depending on the number of pulmonary infiltrates, pain around the lungs during breathing. Disseminated pulmonary mucormycosis with isolated muscular mucormycosis can occur in patients with acute myeloid leukemia.[23]
Disseminated mucormycosis
A disseminated form of mucormycosis with involvement of the cerebellum can occur in patients with multiple myeloma and myelodysplastic syndrome.[24]
Cardiac mucormycosis
It can affect the atria and interventricular septum of the heart characterized by diffuse infiltration into the atrial and interventricular septum of the heart. It can result in death primary due to cardiac failure or secondary due to pulmonary vein involvement resulting in pulmonary infarction.[24]
Renal mucormycosis
It damages the nephrons resulting in loss of renal function and ending in renal failure, a life-threating condition.[24]
Clinical features of mucormycosis
The signs and symptoms of mucormycosis depend on the site of involvement in the body. Rhino-orbital mucormycosis can lead to unilateral pain, unilateral ptosis, facial numbness, discoloration of the skin over the side of the nose, nasal stuffiness, bleeding from the nose (epistaxis), and coughing of blood and puffiness around the eye due to periorbital edema. Orbital apex syndrome is characterized by simultaneous dysfunctions of the cranial nerves, including optic, oculomotor, trochlear, and abducent cranial nerves (resulting in vision loss, ptosis, and ophthalmoplegia external and internal) occurring near the optic canal and superior orbital fissure. Eye redness or pus around the eyeball, sudden unilateral blurriness, or complete loss of vision. Unattended rhino-orbital mucormycosis can also lead to cavernous sinus thrombosis, a life-threatening condition.[24]
STAGES OF MUCORMYCOSIS
The various stages of mucormycosis are shown in Table 1. The complications of mucormycosis described in Table 2.
| Stage | Involvement |
|---|---|
| Stage I | Nose and paranasal sinus |
| Stage II | Nose and paranasal sinus with orbital extension |
| Stage III | Nose, paranasal sinus, and orbit with intracranial extension |
| Types of mucormycosis | Complications |
|---|---|
| Cerebral mucormycosis | Cerebral abscess, cranial palsies, and meningitis |
| Rhino-orbital mucormycosis | Blindness |
| Pulmonary mucormycosis | Hemoptysis and chest pain |
| Gastrointestinal mucormycosis | Gastric perforation |
| Renal mucormycosis | Renal failure |
| Cutaneous mucormycosis | Necrotizing fasciitis |
Diagnostic modalities for mucormycosis
Direct transnasal endoscopy: It reveals a black necrotic eschar tissue that resembles dried blood or a purulosanguinous exudate with halitosis, an important clinical marker in the diagnosis of mucormycosis.[23]
Characteristic red flags/warning signs for mucormycosis
Pain in the maxillary sinus region, impaired or loss of cranial nerve II, III, V, and VI functions, diplopia, proptosis, swelling in the periorbital area, orbital apex syndrome, or a non-healing ulcer involving alveolus and hard palate.[23]
Biopsy of affected tissues
Bronchoalveolar lavage for diagnosis of pulmonary mucormycosis.[23]
Culture
Mucorales are thermotolerant and rapidly grow on any carbohydrate substrate, and colonies appear 24–48 h of incubation. All species of mucormycosis grow well in Sabouraud dextrose agar incubated at 25–30°C. Identification is based on colonial microscopic morphology. About 3% malt extract agar incubated at room temperature is the culture medium for mucormycosis. A major concern with culture is that it has low sensitivity since procedures such as homogenizing and grinding tissue specimens can destroy mucormycetes’ thin, delicate fungal hyphae. Hence, culture is of no value in the diagnosis of mucormycosis. Proper sampling and handling is a pre-requisite and is of major concern for culture.[23]
Molecular methods
Internal transcriber spacer (ITS) region sequencing is a reliable method used for identifying the Mucorales species at molecular level.[23]
Nested polymerase chain reaction (PCR), Real-Time PCR
PCR coupled with electrospray ionization mass spectrometry (PCR/ESI-MS) and PCR/high-resolution melts analysis on deep frozen sections: For detection of Mucorales in the tissues. genes encoding 28 SrDNA, mitochondrial gene rnl, Cot-H gene, cytochrome-b gene, and 18S ribosomal RNA are targets of most molecular methods.[25]
A multiplex PCR method which uses primers that target a small fragments of number of genera/species of Mucorales, or pan fungal primers that target the ITS genomic region[25]
(Mucorgenius®, PathoNostics, Maastricht, the Netherlands). A new pan-Mucorales real-time (qRCR) commercial kit: It is a fast and accurate diagnostic test that was developed after taking a series of blood samples from patients with both invasive mucormycosis and positive results from culture, often several days or weeks before the final diagnosis was made.[23]
Serological tests
Tests for Mucorales cell wall fucomannan detection: Lateral flow immunoassay was demonstrated to be more convenient than Enzyme Linked Immunosorbent Assay (ELISA) and could be used for Bronchoalveolar lavage (BAL), serum, urine, and tissue detection. In murine models, the test was able to both accurately and also identify the following mucorale species Mucor circinelloides, Rhizopus delemar, Cunninghamella bertholletiae, Lichtheimia corymbifera, and post-infection.
An effective tool for monitoring and also diagnosis of invasive mucormycosis in patients with compromised immune systems is serum Mucorales PCR. A major advantage of this test is that it detects 3–68 days earlier than conventional methods. Using a fast probe-based Mucorales-specific real-time PCR (Muc 18S) assay, this method enabled earlier detection of Mucorales in infected tissue and blood samples from patients with invasive mucormycosis and hematological malignancies myeloid leukemia and myeloproliferative disorders like multiple myeloma. They also detect mucormycosis in patients with compromised immune systems earlier than conventional methods, which takes around eight days, or imaging, which takes three days.[23]
Aspergillosis can be distinguished from mucormycosis using immunohistochemistry with monoclonal antibodies against Rhizopus arrhizus (sensitivity: 100%, specificity: 100%).
Gomori methenamine silver stain:
The hyphae of mucormycosis under this stain appear wide 5–20 μm, thin-walled, with few or no septate (Pauci-septae), and broad, ribbon–like and right-angled branching, features characteristic of mucormycosis.[23]
The Periodic Acid-Schiff stain reveals more details of the surrounding tissue than the methenamine silver stain.[23]
Histopathological techniques
Direct fluorescent microscope with potassium hydroxide (KOH) wet mount: In this, the specimen obtained is smeared on a glass slide, and a drop of 20 % KOH is used and mixed with blankophor and calcofluor white covered with a cover glass top and visualized under fluorescent microscope. The fungal hyphae are easily demonstrated by 20% KOH stain. The main disadvantages are that it takes a minimum of 6 weeks to yield a result.[24]
Matrix-assisted laser desorption ionization-time of flight mass spectrometry
A new method for rapid identification of mucormycosis fungus was directly from blood cultures. In this method, the sample to be analyzed is mixed and placed on a plate with a matrix containing the following compounds, namely, 2,5-dihydroxy benzoic acid, and 3,5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid), α-cyano-4-hydroxycinnamic acid, which has high ability of absorbing ultraviolet laser radiation when subjected to exposure from ultraviolet radiation. The generated ions, then, are directed and guided to a mass analyzer.[25]
Metabolomics-breath test
The examination of breath volatile metabolite profiles by Koshy et αl.., using the three Mucorαles species that most commonly cause human Invasive Mucormycosis-R arrhizus var. arrhizus, R. arrhizus var. delemar, and Rhizopus microsporus, by thermal desorption gas chromatography/tandem mass spectrometry.[26]
Sesquiterpene, a volatile metabolite found in the breath of Mucorαles species, could be used to detect infection in vivo in all three species. Using these profiles, one can distinguish between fungal infections and aspergillosis and possibly monitor treatment response without invasive procedures for diagnosing the infections. The main drawback is that it cannot detect low fungal burden infections.[26]
SPECIALIZED IMAGING TECHNIQUES FOR DIAGNOSIS OF MUCORMYCOSIS
Positron emission tomography-computed tomography (CT) with 18-Fluorodeoxyglucose (FDG)
Helpful in identifying the cranial base osteomyelitis due to mucormycosis.[27]
MRI
Black turbinate sign. In contrast to normally enhancing unaffected turbinates, the affected middle and inferior turbinates have a lack of enhancement focally in the affected region, referred to as “the black turbinate sign.” A T1-weighted MRI can show distinct areas of signal hypointensity in the clivus and bilateral petrous apexes of the cranial base, as well as progressive heterogeneity in the central skull base when mucormycosis affects the cranial base. Images showing fat suppression show bilateral thick, smooth enhancement of the dura in the middle cranial fossa and abnormal marrow enhancement in the clivus.[28]
CT
Sinonasal opacification, lack of enhancement of the affected mucosa, obliteration of nasopharyngeal tissue planes, air-fluid concentration, and CT-Lungs usually demonstrate pulmonary nodules >/= to 10, pleural effusion. The “Reverse halo sign” is a characteristic feature seen in Computed Tomography (CT) of lungs in mucormycosis patients. The initial CT scan findings included were a nodule < or > 3 cm or consolidation with surrounding ground-glass radiopacity. The characteristic morphological findings in follow-up CT scans of mucormycosis patients include reverse-halo sign, central necrosis, and air-crescent sign.[29] The main limitation is that while imaging gastrointestinal tract in patients with gastrointestinal mucormycosis can mimic as advanced gastric cancer.[30]
Transthoracic echocardiography
Transthoracic echocardiography is of value in the diagnosis of cardiac mucormycosis, which can present as soft-tissue mass extending into the left atria of the heart or as diffuse infiltration involving both the atria , interventricular septum, and pulmonary vein.[30]
TREATMENT OF MUCORMYCOSIS
Intraoperative frozen section debridement helps to delineate the margins of the infected tissues. Surgical debridement helps penetration of the drug to the site of infection.[31]
Exenteration of the eyeball is indicated when there is a completely affected orbital cavity in rhino-orbital mucormycosis.
Systemic antifungal drug
i.v. Amphotericin B, 5–7.5 mg/kg body weight are preferable. The main concern of i.v. Amphotericin B is it’s nephrotoxicity. Hence, periodic renal function tests such as blood urea nitrogen and serum creatinine must be evaluated every month during the course of therapy.[32]
Liposomal Amphotericin B is suggested whenever there is involvement of the brain, such as cerebrum, resulting in cerebritis, cerebellum as it crosses the blood-brain barrier easily and is less nephrotoxic when compared to Amphotericin B.[32]
Combination antifungal therapy (Polyene-Capsofungin therapy) for mucormycosis revealed significant outcome in patients with rhino-orbital and rhinocerebral mucormycosis.[32]
Posaconazole
Posaconazole indicated only as a salvage therapy for mucormycosis who were refractory or intolerant to polyenesAmphotericin B and not as a first line of a drug to combat mucormycosis.[33]
Isavuconazole
An Food and Drug Administration-approved azole antifungal effective against invasive mucormycosis. This drug inhibits fungal cell membrane synthesis by inhibiting fungal cytochrome P450 lanosterol 14-alpha-demethylase (CYP51), which converts lanosterol into ergosterol. It is available as 100 mg capsule form and 200 mg in a vial. The recommended dosage for mucormycosis is 100 mg capsule twice daily or 200 mg once daily. The intravenous formulation is free from cyclodextrin that is used for facilitating drug solubility. Hence, isavuconazole is free from nephrotoxicity, unlike Amphotericin-B.[34] The complications of antifungal therapy were described in Table 3.
| Antifungal therapy | Complications |
|---|---|
| Amphotericin B/Liposomal Amphotericin B |
Nephrotoxicity. 500–1000 mL of bolus saline must be infused before and after administering Amphotericin. Hyperkalemia |
| Posaconazole | Febrile neutropenia, herpes simplex infection, anemia, arthralgia, back pain, constipation, cough, abdominal pain, diarrhea, and dizziness. |
| Voriconazole | Blurred vision, anaphylaxis, photophobia, skin rashes, and abdominal pain. |
| Isavuconazole | Nausea, vomiting, diarrhea, and abnormal liver function tests. |
Deferasirox
An iron chelator used to treat iron overload caused by iron transfusions acts by having a strong affinity and binding trivalent ferric ions and forming a stable complex, which is eliminated by kidneys. This drug is mainly used after stem cell or allogeneic transplants due to aplastic anemia.[35]
The adjunct therapeutic modalities for mucormycosis
The adjunct ayurvedic therapeutic modalities for mucormycosis described in Table 4. The addition of Ayurveda to conventional allopathic treatment for post-COVID mucormycosis (PCM) Adjunct Ayurveda Therapy + Conventional Allopathic Treatment (AAT + CAT) resulted in a more complete cure of the disease when compared to conventional allopathic therapy alone in terms of symptoms score, disease progression, antifungal medicine requirement, and surgical requirements. PCM patients should consider a combination therapy (AAT + CAT) not only because it is therapeutically effective, but also because it is a safe and economical alternative. Study shows antifungal efficacy and Ayurveda’s role in the management of emergency states, such as epidemics with outbreaks of contagious diseases [Table 4].[36]
| Principle/rationale behind ayurvedic intervention medicine | Name of ayurvedic medicine | Dosage |
|---|---|---|
| Effective against fungal ailments | Gandhaka Rasayana | 500 mg tab thrice a day after food |
| Kaisora Guggulu | 500 mg tab thrice a day after food | |
| Aiding in symptomatic improvement, anti-inflammatory | Dasamula Katutrayadi Kashayam | One gram tablet once a day |
| Vyoshadi Vati | 500 mg 2 tab twice day-chewable | |
| Immune promotive Antihyperglycemic |
Vasanta Kusumakar Ras | One tab once a day |
| Nishamalki | 500 mg 2 tab twice a day | |
| Drugs which improve local hygiene (Topical use) | Surasadi Gana Taila Nasya | 2 drops in each nostril for local application once a day morning |
| Triphala, Daru Haridra Kashaya for Gargling (Kavala) | 50 g of the powder to be boiled in a 400 mL of water and reduced to 100 mL for gargling in mouth with closed lips for 10 min/twice a day (Kavala) | |
| Fumigation of sinuses with herbal Sticks made of Triphala and Daruharidra (Dhuma Nasya) | Fumes from the herbal sticks made of Triphala and Daruharidra powders (Berberis Aristata) |
Complications of mucormycosis
The complications of mucormycosis were enumerated [Table 2].
CONCLUSION
The increase in mucormycosis infection was found to be due to the underlying compromise in the immune system due to an increase in systemic illnesses such as diabetes patients, leukemia, and those under immunosuppressant drug therapy after organ transplantation. Mucormycosis, the Black fungus, is an emerging threat that has led to mortality during the COVID-era. Futuristing point-of-care testing methods like metabolomics-breath test is being evaluated and is promising for early diagnosis of this dreadful mucormycosis infection and enable rapid initiation of treatment.
Ethical approval
Institutional Review Board approval is not required
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Management and outcomes of three cases of rhinocerebral mucormycosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e69-74.
- [CrossRef] [PubMed] [Google Scholar]
- Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-25.
- [CrossRef] [PubMed] [Google Scholar]
- The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15:2-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in the eastern Mediterranean: A seasonal disease. Epidemiol Infect. 2006;134:341-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;1:22-31.
- [CrossRef] [PubMed] [Google Scholar]
- Fatal zygomycosis caused by Mucor indicus in a child with an implantable left ventricular assist device. Pediatr Infect Dis J. 2008;27:365-9.
- [CrossRef] [PubMed] [Google Scholar]
- Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56:29-43.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis outbreak associated with hospital linens. Pediatr Infect Dis J. 2014;33:472-6.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinocerebral mucormycosis: A ten-year single centre case series. Cureus. 2020;29:11776.
- [CrossRef] [PubMed] [Google Scholar]
- Acute subdural hematoma and intracerebral hemorrhage. Rare complications of rhinocerebral mucormycosis. Arch Otolaryngol. 1979;29:279-81.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinocerebral mucormycosis: Pathology revisited with emphasis on perineural spread. Neurol India. 2014;62:383-6.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve palsy: An unusual presentation in patients with rhino cerebral mucormycosis. Indian J Otolaryngol Head Neck Surg. 2019;71:2110-3.
- [CrossRef] [PubMed] [Google Scholar]
- Invasive cutaneous mucormycosis in a preterm neonate presenting as a vesiculobullous lesion. Indian J Pathol Microbiol. 2018;61:103-5.
- [CrossRef] [PubMed] [Google Scholar]
- Peritoneal mucormycosis in a patient receiving continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1989;13:237-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cutaneous and mucosal mucormycosis mimicking pancreatic panniculitis and gouty panniculitis. J Am Acad Dermatol. 2012;66:975-84.
- [CrossRef] [PubMed] [Google Scholar]
- Total gastric necrosis due to mucormycosis: A rare case of gastric perforation. Am J Case Rep. 2018;19:527-33.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric mucormycosis complicated by a gastropleural fistula: A case report and review of the literature. Medicine (Baltimore). 2019;98:18142.
- [CrossRef] [PubMed] [Google Scholar]
- Isolated splenic mucormycosis in a case of aplastic anaemia. BMJ Case Rep. 2018;2018:bcr2017223243.
- [CrossRef] [PubMed] [Google Scholar]
- Primary oral mucormycosis due to Rhizopus microsporus after allogeneic stem cell transplantation. Intern Med. 2018;57:2567-71.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated pulmonary with isolated muscular mucormycosis in an acute myeloid leukemia patient: A case report and literature review. Am J Case Rep. 2019;20:1210-5.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinomaxillary mucormycosis presenting as palatal ulcer: A case report with comprehensive pathophysiology. J Oral Maxillofac Pathol. 2020;24:558-62.
- [CrossRef] [PubMed] [Google Scholar]
- Disseminated mucormycosis with cerebellum involvement due to Rhizomucor pusillus in a patient with multiple myeloma and secondary myelodysplastic syndrome: A case report. Exp Ther Med. 2019;18:4076-80.
- [CrossRef] [PubMed] [Google Scholar]
- Zygomycosis: Conventional laboratory diagnosis. Clin Microbiol Infect. 2009;15:60-5.
- [CrossRef] [PubMed] [Google Scholar]
- Mould routine identification in the clinical laboratory by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. PLoS One. 2011;6:e28425.
- [CrossRef] [PubMed] [Google Scholar]
- Breath-based diagnosis of invasive mucormycosis (IM) Open Forum Infect Dis. 2017;4:53-4.
- [CrossRef] [Google Scholar]
- Imaging of mucormycosis skull base osteomyelitis. AJNR Am J Neuroradiol. 2000;21:828-31.
- [Google Scholar]
- The “black turbinate” sign: An early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;31:771-4.
- [CrossRef] [PubMed] [Google Scholar]
- F-18 fluorodeoxyglucose positron emission tomography/computed tomography image of gastric mucormycosis mimicking advanced gastric cancer: A case report. World J Clin Cases. 2019;26:1155-60.
- [CrossRef] [PubMed] [Google Scholar]
- Cardiac mucormycosis: A case report. Eur Heart J Case Rep. 2019;1:ytz130.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of frozen section analysis for fungal organisms in soft tissue wound debridement margin determination. Diagn Pathol. 2015;10:188.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015;70:3116-23.
- [CrossRef] [PubMed] [Google Scholar]
- Successful posaconazole salvage therapy for rhinocerebral mucormycosis in a child with leukemia. Review of the literature. Rev Iberoam Micol. 2019;36:160-4.
- [CrossRef] [PubMed] [Google Scholar]
- Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: Design, development, and place in therapy. Drug Des Devel Ther. 2018;12:1033-44.
- [CrossRef] [PubMed] [Google Scholar]
- Deferoxamine therapy and mucormycosis in dialysis patients: Report of an international registry. Am J Kidney Dis. 1991;18:660-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of ayurvedic intervention as an adjunct therapy in post COVID-19 mucormycosis (PCM): A non-randomized parallel group study. J Ayurveda Integr Med. 2022;13:100672.
- [CrossRef] [PubMed] [Google Scholar]






