Translate this page into:
Basic histology of hard tissues of teeth using ESEM of ground section and digital drawings

*Corresponding author: Sandhya Tamgadge, Department of Oral and Maxillofacial Pathology, D.Y. Patil University School of Dentistry, Mumbai, Maharashtra, India. sandhya.tamgadge@dypatil.edu
-
Received: ,
Accepted: ,
How to cite this article: Khan A, Tamgadge S. Basic histology of hard tissues of teeth using ESEM of ground section and digital drawings. J Academy Dent Educ. 2025;11:3-9. doi: 10.25259/JADE_24_2024
Abstract
Objectives:
While the histological features of teeth are well-established, there is a continuing need for innovative approaches to visualize and communicate these complex structures effectively, particularly for educational purposes and as a foundation for future research. The objectives of this study were as follows: (1) To integrate environmental scanning electron microscopy (ESEM) imaging with digital drawings to create a novel visual representation of dental hard tissues. (2) To explore the potential of readily available technology (iPad and pencil) for creating accurate integrated histological illustrations of all features together, based on advanced microscopy data. (3) To develop a methodological approach that bridges the gap between microscopic imaging and schematic representations of dental microstructure.
Material and Methods:
A single tooth ground section was examined using ESEM. Additionally comprehensive digital drawings of all hard tissues were created using an iPad and pencil. All the histological features of tooth were also drawn in a single diagram. This combination of techniques allowed for detailed visualization of enamel, dentin, and cementum structures.
Results:
The study produced a series of high-resolution ESEM images coupled with corresponding digital illustrations, providing a unique visual resource for dental histology. While not presenting new ultrastructural findings, the study demonstrates a novel approach to representing known structures.
Conclusion:
This study, while descriptive in nature, introduces an innovative method for visualizing and illustrating dental histology. The integration of ESEM with digital drawing techniques offers a new perspective on dental microstructure, with potential applications in dental education and as a foundation for future research methodologies.
Keywords
Cementum
Dentin
Digital drawings
Enamel
Scanning electron microscopy
INTRODUCTION
Tooth formation involves both ectodermal and ectomesenchymal tissues. The enamel organ, which differentiates from the primitive oral epithelium lining the inner enamel epithelium and stomodeum (early oral cavity), gives rise to enamel, Dentin and pulp originate from the dental papilla, while cementum, periodontal ligament, and alveolar bone develop from the dental follicle[1] [Figure 1].
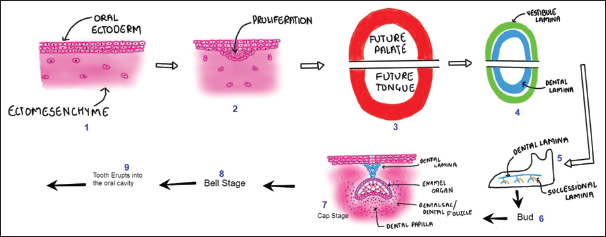
- (Digital drawing) Basic components of odontogenesis.
Tooth development progresses through several distinct morphological stages, named according to the shape of the enamel organ. These are commonly known as the bud, cap, and bell stages [Figure 1].[2] Various microscopic techniques are employed to visualize dental histological structures, including light microscopy, scanning electron microscopy (SEM), and transmission electron microscopy. These methods are crucial for understanding the histological aspects of teeth. To complement these techniques and contribute to the existing literature, digital images were also drawn to enhance comprehension.[3,4]
This study presents the first comprehensive illustration encompassing all histological features of dental hard tissues in a single, integrated depiction. By combining advanced microscopy with digital artistry, we offer a unique visual synthesis of tooth microstructure that has not been previously represented in this manner. This novel approach provides a unified perspective on the intricate relationships between enamel, dentin, and cementum, potentially enhancing understanding and facilitating more intuitive learning of dental histology.
While the previous studies have utilized digital illustrations to depict various aspects of dental histology, our work represents a significant advancement in the comprehensive visualization of tooth microstructure. Unlike earlier efforts that focused on isolated features or specific dental tissues, this study is the first to our knowledge to synthesize all histological elements of dental hard tissues into a single, cohesive digital illustration too.
This unified depiction not only consolidates existing knowledge but also provides a novel perspective on the spatial relationships and structural continuity of dental hard tissues. By bridging the gap between isolated microscopic observations and overall tooth architecture, our work offers a more intuitive understanding of dental histology, potentially enhancing both research visualization and educational resources in the field of dentistry.
“SEM utilizes a focused beam of electrons to scan the surface of dental specimens, producing high-resolution images with exceptional depth of field. This technique allows for detailed visualization of tooth microstructure at magnifications far beyond the capabilities of light microscopy, revealing intricate features of enamel, dentin, and cementum at the submicron level. SEM has become an invaluable tool in dental histology, enabling researchers to examine the ultrastructure of dental tissues with unprecedented clarity.
“Environmental SEM (ESEM) offers distinct advantages for examining dental hard tissues. Unlike conventional SEM, ESEM allows for the observation of hydrated, non-conductive specimens without the need for complex sample preparation or conductive coatings. This preservation of the natural state of dental tissues provides more accurate representations of their in vivo characteristics, particularly beneficial for studying the organic components and water-dependent structures within teeth.”[5-7] Therefore, the aim of the study was to correlate ESEM analysis of a single ground section of tooth, combined with digital illustrations, to enhance our understanding of the microstructure and histological features of dental hard tissues compared to traditional histological methods such as ground section.”
MATERIAL AND METHODS
This study examined a 2 mm-thick ground section of an unfixed human tooth using an ESEM at ICON LABS PVT LTD in Mumbai.
ESEM procedure
The unfixed tooth section was trimmed with a lathe machine and directly observed under the ESEM microscope without any specimen preparation. We conducted comprehensive scanning of all areas and captured relevant images.
The authors also created digital illustrations depicting all observed histological features. One of the images had all the histological structures in one image depicting ground section of a tooth [Figures 1 and 2].
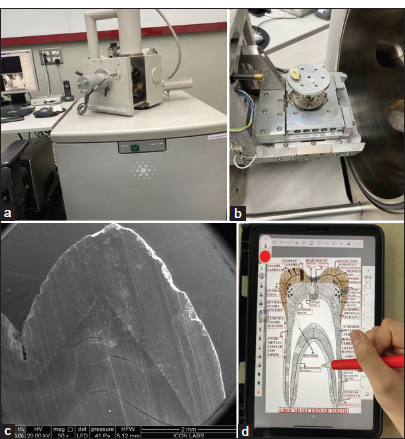
- (a and b) Environmental Scanning electron microscope (ESEM), (c) ESEM of tooth 50X, and (d) iPad and pencil used for digital drawings to integrate all histological features into one image.
Digital illustrations
Digital images were created using an iPad (5th generation) and Apple Pencil (2nd generation). All the histological features of hard tissues of tooth were compiled into 1 image to understand the correlation of all the histological structures.
RESULTS
The ESEM functions by scanning the surface of a sample with a focused beam of electrons, generating images with exceptionally high magnification. Through the utilization of this advanced technology, a concise comprehension of the histology of enamel, dentin, and cementum was achieved.
Following features were observed-
Enamel (ESEM and digital drawings)
The enamel serves as the protective covering across the entire surface of the tooth crown in comprising both organic and inorganic components. The inorganic matter constitutes 96%, primarily composed of hydroxyapatite crystals, while the organic material makes up the remaining 4%.
Ground section provided optimal visualization of hard tissues [Figure 3].
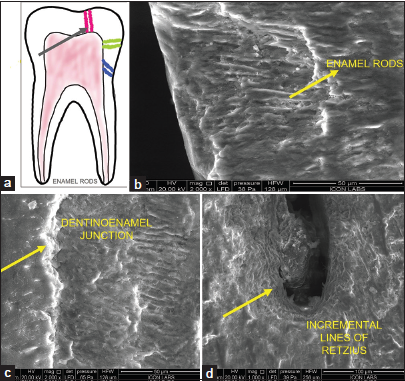
- (a) (Digital drawing) different directions of enamel rods (black arrow), (b) enamel rods ESEM, (c) dentinoenamel junction, and (d) incremental lines of enamel (b,c,d- structures are denoted by yellow arrows). ESEM: Environmental scanning electron microscope.
In longitudinal section, enamel rods displayed a distinctive keyhole shape, as well as parallel structures. The dentinoenamel junction (DEJ) appeared scalloped at ×400 magnification, with enamel convexities projecting onto the dentin at ×2000 magnification [Figure 3]; similar structures were digitally drawn.
Dentin (ESEM features and digital drawings)
Dentinal tubules, observed as hollow tubes extending from the pulp to the outer dentin surface, exhibited a distinctive curved or sigmoid course. Peritubular dentin intertubular dentin and lateral branches were clearly evident [Figure 4a-d]. Above features were explained through digital drawings too including additional features of dentinal tubules.
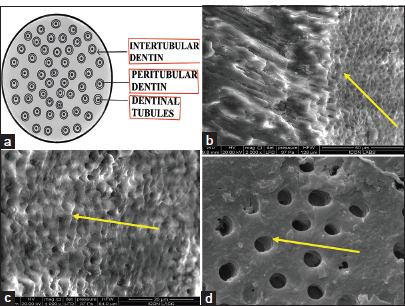
- (a) (Digital drawing) T. S of dentinal tubules, (b) scanning electron microscope of L. S and T. S of dentinal tubules, (c) T.S of dentinal tubules, and (d) T.S of dentinal tubules. T.S: Transverse section, L.S: Longitudinal section (b.c,d- structures are denoted by yellow arrows).
Cementum (ESEM features and digital drawings)
The embedded ends of PDL fibers in the cementum, known as Sharpey’s fibers, were prominently visible as horizontal clefts along the length of the cemental layer. Incremental lines of Salter were also distinctly evident as parallel lines.
Acellular cementum covered the cervical half of the root, while cellular cementum was present in the apical third with [Figure 5].

- (a) (Digital drawing) showing Sharpeys fibers in cementum, (b) Environmental scanning electron microscope (ESEM) Sharpeys fibers in cementum, (yellow arrow) and (c) SEM Sharpeys fibers and acellular cementum (yellow arrow).
Cementoenamel Junction (CEJ)
The CEJ is the junction where the enamel of the crown intersects with the cementum of the root [Figure 6].
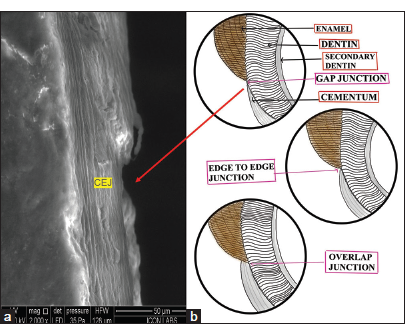
- (a) Scanning electron microscope of gap type cementoenamel junction denoted by red arrow (CEJ) in cementum and (b) (Digital drawing) showing three types of CEJ.
In our observation, the junction exhibited the gap type configuration. All three junctions were explained through digital drawings.
DISCUSSION
The present study employed a multi-modal approach, integrating ESEM, of a ground section of a single tooth, and digital drawings, to provide a comprehensive understanding of the intricate histological features of dental hard tissues. This collaborative efforts of authors, involving hands-on research in the SEM laboratory and digital illustration, offers a unique perspective on the structural components and their interrelationships within enamel, dentin, and cementum. The field of dental research has made significant strides in understanding structural aspects of dental tissues.[8,9] This report aims to synthesize and analyze findings from ESEM research and digital drawings on histological features of tooth using ground sections and correlating with the previous studies.
ESEM represents a significant advancement over traditional SEM in the field of dental histology research. While both techniques provide high-resolution imaging of surface topography, ESEM offers distinct advantages for examining biological specimens, particularly dental tissues.[3,10] Unlike conventional SEM, which requires samples to be conductive and observed under high vacuum conditions, ESEM allows for the examination of non-conductive, hydrated specimens in their natural state. This is achieved through the use of a variable pressure chamber and specialized detectors that can operate in a gaseous environment. The ability to maintain samples in a hydrated state is particularly beneficial for dental tissues, as it preserves their native structure and prevents artifacts associated with dehydration and conductive coating. Furthermore, ESEM’s capacity to function at higher pressures and lower voltages reduces the risk of beam damage to sensitive dental structures. This technique also facilitates dynamic studies, such as observing mineralization processes or the effects of various treatments on dental tissues in real-time. These capabilities make ESEM an invaluable tool for comprehensive analysis of dental hard tissues, offering insights that complement and extend beyond those obtainable through traditional SEM or light microscopy techniques.[5,6] Therefore, we could examine the ground section of tooth directly under microscope without any processing steps.
Enamel histology has been extensively studied using ESEM, including investigations of various lesions such as incipient erosion and fluorosis.[11-13] Enamel, the tooth crown’s outermost layer, is characterized by its highly mineralized composition and unique prismatic structure. ESEM micrographs of ground sections revealed an organized arrangement of enamel rods or prisms, with their long axes perpendicular to the DEJ.[14,15] Enamel surface toward DEJ showed projections to interdigitate with dentin.[16-18]
The structural properties of Retzius lines in human dental enamel was examined by Risnes. This study adds to our understanding of dental histology by shedding light on the process of enamel development. We also observed similar lines prominently near fissure area.[16] Dentin, the bulk of the tooth, exhibited a complex network of dentinal tubules surrounded by a highly mineralized matrix.[19] The ESEM images of ground section provided a detailed visualization of the intricate organization and branching patterns of these tubules, which extend from the pulp chamber toward the DEJ.[14,20,21] Dentin surface to DEJ showed concavities in which enamel projections were interdigitating.[22-26] Pulpal wall of dentin was concave showing T.S of dentinal tubules. Dentin has been investigated under SEM by various researchers.
Tamgadge et al. offer insights into the role of odontoblasts in health and disease, presenting a three-dimensional histological perspective. The study enhances our understanding of the cellular processes involved in maintaining dental health.[27]
Dentin normal histology has been studied widely and in various lesions.[28-32]
The cementum, a specialized calcified tissue covering the root surface, exhibited a distinct layered structure in the SEM images and ground section [Figure 4]. The acellular cementum, closest to the dentin, comprised a fibrillar matrix interspersed with Sharpey’s fibers, facilitating attachment to the periodontal ligament.[12,14,33,34] The cementodentinal junction, where the acellular cementum merges with the dentin, was clearly visible [Figure 5], highlighting the intricate interface responsible for anchoring the tooth to the alveolar bone and resisting masticatory forces. Sharpey’s fibers in Cementum have been studied by[35-38] we found incremental lines, gap junction, cementodentinal junction, etc., similar features were also recreated with the help of digital drawings.[4,14]
The digital drawings [Figure 2d], created using an iPad, provided simplified yet informative compiled integrated representations of the histological features observed through SEM and the ground section. These illustrations facilitated a clear understanding of the spatial relationships and organizational patterns of the various dental hard tissue components, complementing the microscopic observations.
Literature on digital drawings is sparse. Our preliminary attempt will add data in the literature.
In summary the amalgamation of these diverse studies provides a comprehensive overview of the current state of dental research. These findings collectively contribute to advancing our understanding of oral health and diseases. This document serves as a valuable resource for researchers, clinicians, and educators in the field of dentistry, fostering continued exploration and innovation.
Limitations
Regarding the use of a single tooth specimen for ESEM, this was indeed a limitation of our current study. In future work, we plan to expand our sample size to provide a more comprehensive analysis and to explore variations in dental histology across different tooth types and conditions.
Significance
This work contributes to the field by introducing a new approach to dental histology visualization, potentially enhancing understanding and teaching of these structures. It also lays the groundwork for future studies that could apply this technique to examining pathological changes or effects of various treatments on dental tissues.
Highlight of the study
While we acknowledge that the basic histological features of teeth are well-established, our study aims to contribute in several unique ways:
Novel visualization approach: Our study combines ESEM imaging with digital drawings created using an iPad and digital pencil. This innovative approach bridges the gap between traditional microscopic imaging and schematic representations, offering a new perspective on dental histology that can enhance understanding and teaching of these structures.
Educational value: It offers students and practitioners a clearer, more intuitive understanding of dental microstructure.
By integrating all features into one illustration, it facilitates a holistic understanding of dental structures and their interrelationships.
CONCLUSION
This study demonstrates the significant potential of combining readily available digital technology with advanced microscopy techniques to create comprehensive, integrated illustrations of dental hard tissues. The accessibility of the digital illustration method, combined with the powerful analytical capabilities of ESEM, suggests great potential for widespread adoption in dental histology studies. This innovative technique could revolutionize how dental histology is taught, studied, and communicated, opening new avenues for interdisciplinary collaboration and inspiring similar innovations in other areas of biomedical science. As dental education continues to evolve in the digital age, integrated approaches like this one, which combine traditional knowledge, modern technology, and advanced microscopy, promise to play a crucial role in shaping the future of dental histology visualization and comprehension.
Acknowledgment
Authors would like to acknowledge “Wolters Kluwer India Pvt. Limited” for funding the laboratory charges of this research.
Ethical approval
Institutional Review Board approval is not required for this in vitro study involving ESEM analysis of a single anonymized ground section sample.
Declaration of patient consent
Patient’s consent not required as there are no patients involved in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Orban’s oral histology and embryology (13th ed). New Delhi: Elsevier; 2010. p. :50-92.
- [Google Scholar]
- Oral development and histology (3rd ed). United States: Theime Publisher; 1988. p. :298-332.
- [Google Scholar]
- Bancroft’s theory and practice of histological techniques. (8th ed). China: Elsevier; 2013. p. :1-537.
- [Google Scholar]
- Difiore’s atlas of oral histology with functional correlations (11th ed). United States: Lippincot Williums and Wilkins-Wolters and Kluwers; 2005. p. :1-23.
- [Google Scholar]
- Advantages of environmental scanning electron microscopy in studies of microorganisms. Microsc Res Tech. 1993;25:398-405.
- [CrossRef] [PubMed] [Google Scholar]
- Ultramicrotomy in the ESEM, a versatile method for materials and life sciences. J Microsc. 2009;233:140-8.
- [CrossRef] [PubMed] [Google Scholar]
- ESEM detection of foreign metallic particles inside ameloblastomatous cells. Ultrastruct Pathol. 2015;39:329-35.
- [CrossRef] [PubMed] [Google Scholar]
- The theory and practice of histological techniques In: Pathology. Vol 24. United Kingdom: Churchill Livingstone; 1992. p. :320.
- [CrossRef] [Google Scholar]
- Histology laboratory manual. Available from: http://www.columbia.edu/itc/hs/medical/sbpm_histology_old/histology/histology_stain2.html [Last accessed on 2024 May 01]
- [Google Scholar]
- Outline and arrangement of enamel rods in human deciduous and permanent enamel. 3D-reconstructions obtained from CLSM and SEM images based on serial ground sections. Eur J Oral Sci. 2001;109:409-14.
- [CrossRef] [PubMed] [Google Scholar]
- The macroscopic and scanning electron-microscopic appearance and microhardness of the enamel, and the related histological changes in the enamel organ of erupting sheep incisors resulting from a prolonged low daily dose of fluoride. Arch Oral Biol. 1988;33:361-73.
- [CrossRef] [PubMed] [Google Scholar]
- The use of scanning electron microscopy in studying enamel caries. Scanning Microsc. 1987;1:1109-23.
- [Google Scholar]
- Textbook of oral embryology and histology (2nd ed). New Delhi: Jaypee Brothers Medical Publishers; 2023. p. :366.
- [Google Scholar]
- A scanning electron microscope study of the three-dimensional extent of Retzius lines in human dental enamel. Eur J Oral Sci. 1985;93:145-52.
- [CrossRef] [PubMed] [Google Scholar]
- Structural characteristics of staircase-type retzius lines in human dental enamel analyzed by scanning electron microscopy. Anat Rec. 1990;226:135-46.
- [CrossRef] [PubMed] [Google Scholar]
- Normal enamel. II. Qualitative polarized light study. J Dent Res. 1966;45:261-5.
- [CrossRef] [PubMed] [Google Scholar]
- Scanning electron microscopy (SEM) methods for dental enamel. Methods Mol Biol. 2019;1922:293-308.
- [CrossRef] [PubMed] [Google Scholar]
- Use of optical microscopy for evaluation of tooth structure. J Achiev Mater Manuf Eng. 2016;79:31-40.
- [CrossRef] [Google Scholar]
- Comparative observations on the spacing of short-period (von Ebner's) lines in dentine. Arch Oral Biol. 1998;43:1009-21.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional ultrastructural analysis of the interface between an implanted demineralised dentin matrix and the surrounding newly formed bone. Sci Rep. 2018;8:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative scanning electron microscopic study of the effect of different dental conditioners on dentin micromorphology. J Appl Oral Sci. 2008;16:100-5.
- [CrossRef] [PubMed] [Google Scholar]
- Ten cate's oral histology: Development, structure, and function In: Ten cates oral histology development structure and function. Netherlands: Elsevier; 2012. p. :1-379.
- [Google Scholar]
- Thin sections for hard tissue histology: A new procedure. J Microsc. 2000;199:244-7.
- [CrossRef] [PubMed] [Google Scholar]
- Dentin: Structure, composition and mineralization. Front Biosci (Elit Ed). 2011;3:711-35.
- [CrossRef] [PubMed] [Google Scholar]
- Odontoblasts in health and disease with an additional note on its three-Dimensional histological perspective. J Sci Soc. 2023;50:4.
- [CrossRef] [Google Scholar]
- Demineralized dentin 3D porosity and pore size distribution using mercury porosimetry. Dent Mater. 2009;25:729-35.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between large tubules and dentin caries in human deciduous tooth. Bull Tokyo Dent Coll. 2005;46:7-15.
- [CrossRef] [PubMed] [Google Scholar]
- Qualitative and quantitative assessment of intratubular dentin formation in human natural carious lesions. Calcif Tissue Int. 2001;69:268-73.
- [CrossRef] [PubMed] [Google Scholar]
- Coronal dentinal tubules of non-erupted deciduous incisors. Pesqui Odontol Bras. 2002;16:12-7.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased dentin tubules density and reduced thickness of peritubular dentin in hyperbilirubinemia-related green teeth. J Clin Exp Dent. 2017;9:e622-8.
- [CrossRef] [PubMed] [Google Scholar]
- Histology of human cementum: Its structure, function, and development. Jpn Dent Sci Rev. 2016;52:63-74.
- [CrossRef] [PubMed] [Google Scholar]
- Study of the microstructure of mineralised tissues in 50 human primary teeth. Int J Oral Maxillofac Pathol. 2014;5:12-8.
- [Google Scholar]
- Scanning electron microscopy of cementum and sharpey fibre bone. Z Zellforsch Mikrosk Anat. 1968;548:536-48.
- [CrossRef] [PubMed] [Google Scholar]
- Scanning electron microscopy of the apical structure of human teeth. Ultrastruct Pathol. 2007;31:321-5.
- [CrossRef] [PubMed] [Google Scholar]
- Scanning electron microscopy of incinerated dental tissues. J Can Soc Forensic Sci. 1978;11:203-10.
- [CrossRef] [Google Scholar]
- Surface morphology changes in human enamel, dentin and cementum following bleaching: A scanning electron microscopy study. Endod Dent Traumatol. 1996;12:82-8.
- [CrossRef] [PubMed] [Google Scholar]







