Translate this page into:
Oral squamous cell carcinoma: A case report with scanning electron microscopy, Mallory’s, Masson’s trichrome, orcein, and Papanicolaou staining

*Corresponding author: Sandhya Tamgadge, Professor, Department of Oral Pathology, D.Y. Patil University School of Dentistry, Mumbai, Maharashtra, India. sandhya.tamgadge@dypatil.edu
-
Received: ,
Accepted: ,
How to cite this article: Manishankar S, Tamgadge S, Pereira T, Tamgadge A. Oral squamous cell carcinoma: A case report with scanning electron microscopy, Mallory’s, Masson’s trichrome, orcein, and Papanicolaou staining. J Academy Dent Educ. 2024;10:47-50. doi: 10.25259/JADE_49_2023
Abstract
Squamous cell carcinoma is a malignant epithelial tumor with squamous differentiation. It is characterized by formation of keratin or presence of intercellular bridges. This neoplasm is also known as epidermoid carcinoma and is the second most common malignant neoplasm of the oral cavity. This neoplasm is seen more frequently in the lower lip, tongue, floor of the mouth, upper lip, buccal mucosa, gingiva, and in some cases, in the hard palate and maxillary sinus. Multiple reports on oral squamous cell carcinoma (OSCC) are found in the literature but very few report the various investigations done using special stains and scanning electron microscopy. There is a need for investigations using special stains as it is less expensive and may also help in identifying various characteristics of OSCC. Here, we present a case report of well-differentiated squamous cell carcinoma in a 60-year-old male patient with complaints of pain and ulcers on both cheeks for 2–3 months.
Keywords
Scanning electron microscopy
Masson’s trichrome stain
Mallory’s stain
Squamous cell carcinoma
Papanicolaou
Orcein
INTRODUCTION
Oral cancer is one of the most common cancers in India.[1] Oral squamous cell carcinoma (OSCC) accounts for 95% of all head-and-neck cancers.[2] This neoplasm is more commonly seen in men over the age of 40 years.[3] Squamous cell carcinoma is generally seen in tobacco-consuming patients.[4] Histologically, OSCC, like other epithelial malignancies, is characterized by abnormal cell division, invasion of malignant epithelial cells into connective tissue, and aberrant keratinization, especially in the form of keratin pearls.[5] The various reports available generally focus on the etiopathogenesis or treatment modalities and various immunohistochemistry (IHC) markers of OSCC. Some articles on gene research are also available, but very few report on the compiled investigations of scanning electron microscopy (SEM) and special stains. Special stains such as Mallory’s stain, van-Gieson stain, Feulgen stain, Papanicolaou (PAP) stain, Masson’s trichrome, and crystal violet were used for investigation of OSCC in previous studies, which generally gave a common inference that these stains can be used as adjuncts in the diagnosis of OSCC. Normal microscopes usually provide a magnification of ×40–×1,000 but using SEM, a magnification of ×1,00,000 can be achieved.[6] A compiled report on the mentioned stains and SEM is rare in the literature. This report is a case of OSCC in the gingiva of a 60-year-old patient with additional investigations done using Malloy stain, Masson’s trichrome, orcein, PAP staining findings, and SEM.
METHODOLOGY
A case of well-differentiated squamous cell carcinoma was evaluated using H&E, special stain, and SEM. Special stain was used to analyze the features of neoplastic epithelial cells. These stains were PAP, Mallory’s, Masson’s trichrome, and orcein stain. SEM analysis was performed in Icon Labs Pvt. Ltd., Icon House, Sanpada, Mumbai, for which unstained paraffin-embedded section was directly observed under Quanta 200 ESEM system microscope.
CASE REPORT
Ethical approval was not considered as a patient’s identity was not revealed. A 60-year-old male patient reported to the Department of Oral Pathology, DY Patil University School of Dentistry, Nerul, Navi Mumbai, with a chief complaint of ulcer and pain on both cheeks . On intraoral examination, a white patch was observed on the left buccal mucosa and ulcerative growth was observed on the right buccal mucosa, both extending from the occlusal level to the lower buccal sulcus. The patient was a heavy smoker who smoked 10–15 bidis per day for the past 40 years. An incisional biopsy was taken, and it showed the following features. Hematoxylin and eosin stain section shows orthokeratinized stratified squamous epithelium of variable thickness with few areas of keratin plugging. It was highly proliferative, leaving strands and cords in the connective tissue. It showed areas of individual cell keratinization and keratin pearl formation. The cells were hyperchromatic and pleomorphic, with an increased nuclear-cytoplasmic ratio. There is a break in the continuity of the basement membrane. Underlying connective tissue shows areas of epithelial keratinization with dense inflammatory cells infiltrated. A few islands of proliferating epithelial cells were also seen. Although H&E staining is enough for diagnosis, to add to the literature, a few special stains were also used, such as Mallory’s stain, Masson’s trichrome, PAP, and orcein. SEM was also performed. Mallory’s stain and Masson’s trichrome stain revealed invading malignant islands with keratin pearls. PAP stain showed positive malignant cells with dark blue to black and dysplastic features. SEM showed keratin accumulations in the form of whorls surrounded by tumor microenvironment. As the patient was from a low socioeconomic background and from a remote place, he lost follow-up despite repeated efforts [Figures 1 and 2].

- (a) Ulcerative growth on right buccal mucosa (Black arrow). (b) Leukoplakic patch on left buccal mucosa (Black arrow). (c) Environmental scanning electron microscope (ESEM) of oral squamous cell carcinoma (OSCC) showing keratin pearl (Black arrow). (d) Hematoxylin & Eosin (H&E) staining of OSCC (Black arrow).
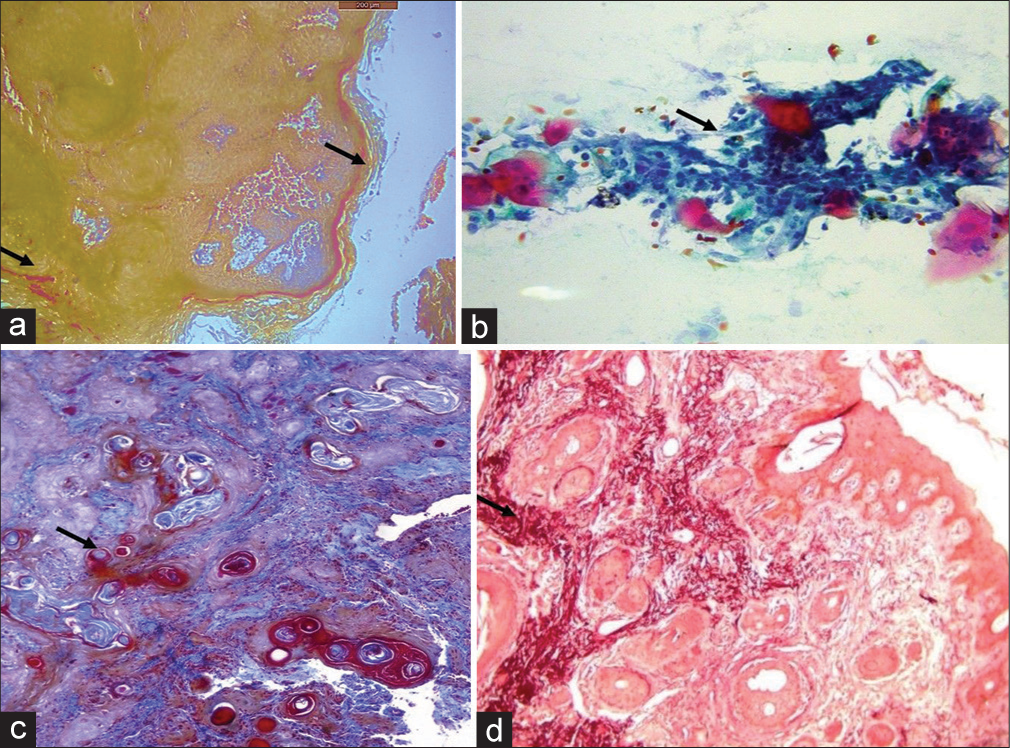
- (a) Mallory’s stain shows keratin staining on the surface and within the stroma (Black arrow). (b) Papanicolaou stain of the smear shows neoplastic cells (Black arrow). (c) Masson’s trichrome stain shows invaded neoplastic islands (Black arrow). (d) Shikata’s modified orcein stain shows elastic fibers around the epithelial islands (Black arrow).
DISCUSSION
There are many different methods for the diagnosis of OSCC, but tissue biopsy and histopathological examination should be the gold standard for diagnosis.[7] OSCC is generally classified into three different types based on the degree of differentiation well differentiated, moderately differentiated, and poorly differentiated.[5] The degree of differentiation is associated with the recurrence and metastasis rates of OSCC.[8] The purpose of this study was to observe the various characteristics of OSCC when different stains were used. In this case report, the techniques used were SEM, Mallory’s, Masson’s trichrome, orcein, and PAP staining. Our patient had a severe tobacco addiction and according to multiple studies, he had a high risk of OSCC which is strongly evident based on the observed clinical features.[4,9] A previous study by Mohanta et al. reported that the site-specific pattern of keratinization can be used for the early detection of OSCC.[10] In our patient, H&E slides revealed keratin accumulation in a specific area in the form of keratin whorls. In the case of SEM, a higher magnification is achieved (×100,000 as compared to ×1,000), which allows us to observe more detailed features of OSCC. The keratin whorls surrounded by the tumor microenvironment give us strong evidence of OSCC, which is generally seen as an area of keratinization in H&E-stained slides. Thus, SEM supports the diagnosis in this case. Another finding seen is the loss of tissue structure. In a previous study using electron microscopy, Leek and Albertsson observed a decrease in a number of desmosomes, leading to loss of structure.[11] Based on these findings, it can be diagnosed as a well-differentiated OSCC. The presence of dense inflammatory cell infiltrate supports the same.[12] Mallory stain is generally used for studying keratin. The current case showed presence of malignant islands of cells surrounded by keratin. This may help us in identifying the potential of the tumor to metastasize. Dysplastic features of positive malignant cells were seen in the PAP. Different elastic fiber patterns are seen in OSCC.[13] Orcein-stained slides showed a protective cuff of elastic fibers around the malignant epithelial cells, which help in the invasion. Rivera and Venegas concluded that differentiation in OSCC is associated with cancer-affected fibroblasts.[14] These findings may help us in identifying the pattern of the cells present in the tumor. Masson’s trichrome is a simple and cost-effective method for the evaluation of collagen fibers.[15] Anatomic predilection site and association with classic risk factors vary with age.[16] According to their study, in an elderly patient, the alveolar process is affected more. However, our case does not comply with their findings as OSCC is seen in the buccal mucosa. Mallory stain will detect orange-colored concentric layers of keratin around the invaded neoplastic islands.[17] Orcein stain detects elastic fibers around the invading islands. The number of elastic fibers decreases as the grade of malignancy advances as per the literature. We saw a large number of fibers around keratin pearls.[13] PAP stain is used to detect the grade of dysplasia of epithelial squamous in cytological smears. Malignant cells will show blue to black enlarged nucleus as compared to normal cells which show light pink to bluish cytoplasm. It truly reflects the degree of differentiation of squamous cells.[18]
CONCLUSION
Special stains are less expensive and can easily locate high grades of OSCC. Poorly and undifferentiated OSCC are difficult to diagnose using H&E stains, sometimes highly dysplastic cells. Special cells can impart specific colors to the non-cohesive undifferentiated dysplastic invaded cells. Multiple reports of OSCC in western populations are available, but very few on the Indian population. Therefore, this report with multiple special stains and SEM will add more data to the literature.
Acknowledgment
The authors are grateful to the senior laboratory technician of the Department of Oral Pathology and Microbiology, Mrs. Rajshree Dahale, D. Y. Patil University School of Dentistry, Nerul, Navi Mumbai, for contributing to staining procedures.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The epidemiology of mouth cancer: A review of global incidence. Oral Dis. 2000;6:65-74.
- [CrossRef] [PubMed] [Google Scholar]
- Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765-81.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-pathological features of squamous cell carcinoma of the oral cavity in patients <40 years of age. J Oral Pathol Med. 2005;34:129-33.
- [CrossRef] [PubMed] [Google Scholar]
- Etiological factors in oral squamous cell carcinoma. Community Dent Oral Epidemiol. 1977;5:301-6.
- [CrossRef] [PubMed] [Google Scholar]
- Shafer's textbook of oral pathology (5th ed). New Delhi: Elsevier Ltd; 2006. p. :404-7.
- [Google Scholar]
- Scanning electron microscopy: An introduction. III-Vs Rev. 2000;13:40-4.
- [CrossRef] [Google Scholar]
- Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Med Oral Patol Oral Cir Bucal. 2015;20:e305-15.
- [CrossRef] [PubMed] [Google Scholar]
- Dentin reactions to caries are misinterpreted by histological “gold standards”? F1000Res. 2014;3:13.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiological trends of oral squamous cell carcinoma-an institutional study. Muller J Med Sci Res. 2021;12:1.
- [CrossRef] [Google Scholar]
- Pattern of keratinization in oral squamous cells during carcinogenesis. IOSR J Dent Med Sci. 2014;13:83-91.
- [CrossRef] [Google Scholar]
- Electron microscopy of squamous cell carcinoma of the head and neck. Scanning. 2000;22:326-31.
- [CrossRef] [PubMed] [Google Scholar]
- Shafer's text book of oral pathology scanned by camscanner Netherlands: Elsevier; 2006. p. :567-658.
- [Google Scholar]
- Morphological analysis of elastic fibers in various grades of oral squamous cell carcinoma and epithelial dysplasia using Verhoeff-Van Gieson stain. Rambam Maimonides Med J. 2019;10:e0014.
- [CrossRef] [PubMed] [Google Scholar]
- Histological and molecular aspects of oral squamous cell carcinoma. Oncol Lett. 2014;8:7-11.
- [CrossRef] [PubMed] [Google Scholar]
- A quantitative and qualitative comparative analysis of collagen fibers to determine the role of connective tissue stroma in oral squamous cell carcinoma using special stains and polarized microscopy. J Oral Maxillofac Pathol. 2020;24:398-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic features of oral squamous cell carcinoma: Do they vary in different age groups? J Oral Maxillofac Surg. 2014;72:1291-300.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of feulgen, crystal violet and Mallory's PTAH stains in assessment of mitotic figures in 3 grades of oral squamous cell carcinoma. Oral Maxillofac Pathol J. 2020;11:53-6.
- [Google Scholar]
- Premalignant and malignant lesions of oral cavity in eastern India: A hospital-based study. Eur J Cancer Prev. 2021;30:393-9.
- [CrossRef] [PubMed] [Google Scholar]






