Translate this page into:
Assessment of the odontoclastic activity in extracted deciduous teeth with and without dental caries

*Corresponding author: Vinola Duraisamy, Professor and Head, Department of Pediatric and Preventive Dentistry, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India. drvinola@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Priya SV, Duraisamy V, Sekar B. Assessment of the odontoclastic activity in extracted deciduous teeth with and without dental caries. J Academy Dent Educ 2023;9:13-7.
Abstract
Objectives:
The aim of the study is to assess the odontoclasts and inflammatory cells in extracted deciduous teeth, with and without dental caries, and thereby evaluate the possibility of decreased rate of resorption/delay in exfoliation due to alteration in cell count of odontoclasts.
Material and Methods:
Twenty extracted deciduous teeth (10 with dental caries and 10 without dental caries) were collected, prepared, sectioned, and stained to study under a light microscope with a magnification of ×400. Histological analysis was done to assess the number, shape, and nature of odontoclasts and inflammatory cells.
Results:
The findings showed that the odontoclastic activity is more in non-carious deciduous teeth than in carious deciduous teeth which was statistically significant (P < 0.05). Findings also indicate the predominance of inflammatory cells and evident hyperemia in carious deciduous teeth which was highly significant.
Conclusion:
Odontoclast number and activity are predominant in non-carious teeth whereas there is a clear dominance of inflammatory cells in the carious specimen over the non-carious specimen which establishes that dental caries in deciduous teeth may influence the normal physiologic resorption at the cellular level with reduced odontoclast activity, reducing the rate of resorption and delaying exfoliation.
Clinical Significance:
This study supports the fact of delayed exfoliation/shedding in carious deciduous teeth.
Keywords
Odontoclasts
Inflammatory cells
Caries
Deciduous teeth
Resorption
INTRODUCTION
Primary teeth differ from permanent teeth by their nature to undergo physiologic resorption, thereby working toward their own shedding. Exfoliation of deciduous teeth is an essential and indisputable action by nature. The process of resorption may be defined as “a condition associated with either a physiologic or a pathologic process resulting in the loss of dentin, cementum, and/ or bone.”[1] The cells of resorption, that is, odontoclasts, osteoclasts, and fibroblasts function more actively, as the period of exfoliation/shedding is being approached. Over this phase of time, a substantial amount of changes is seen to occur in these cells and the surrounding environment.
In recent years, several studies have given an in-depth analysis of the pulp, during its resorptive phase and its correlation with the concerned immune cells.[2] During the episode of physiologic resorption, odontoclasts appear to be originating from the wall of the pulp chamber and hollowing out the tooth from within. Nevertheless, supporting the statement of Harndt and Zerosi, Furseth[3] in his study stated that in the case of human primary dentition where the normal pulp structure is preserved until its exfoliation, the pulp does not play a part in physiologic resorption. The documentation of time-related histologic changes in the pulp during this resorption process, given by Yildirim et al., has shown that the odontoclastic resorption of coronal dentin takes place just before shedding.[4] The pattern of resorption by multi-nucleated cells is seen to proceed from predentin to dentin, following the infiltration of inflammatory cells into the pulp and degeneration of odontoblasts.
Sahara et al., in 1994 showed, this internal resorption is always initiated from the predentin surface and proceeds to the dentine. The previous studies indicate that the resorption can occur when the protective unmineralized tissues such as pre-cementum and pre-dentin are breached or mechanically damaged, allowing osteoclasts/odontoclasts to gain access to calcified dental tissues.[5] At the terminal stages of resorption, odontoclasts gradually disappear, and the resorbed dentine surface is repaired by cementum-like tissue deposition. Although, in the case of internal resorption, this resorption-repair cycle can be witnessed only once, until the teeth are exfoliated.[6]
The present study aimed to assess the odontoclasts and inflammatory cells in extracted deciduous teeth, with and without dental caries, and thereby evaluate the possibility of decreased rate of resorption/delay in exfoliation due to alteration in cell count of odontoclasts. To the best of our knowledge, there are no other studies that evaluate the histological and numerical changes occurring in the odontoclasts and inflammatory cells in deciduous teeth affected by dental caries. The influence of dental caries over these resorptive functions occurring before shedding and the corresponding sequelae of changes are to be analyzed. Histological and histochemical observations are done to compare and contrast these odontoclastic changes i.e. shape and number. Further, in the retained deciduous tooth, the possibility of decreased rate of resorption/delay in exfoliation, due to alteration in the cell count of odontoclasts is evaluated.
MATERIAL AND METHODS
Experimental material
Twenty extracted retained deciduous teeth from patients 8–13 years of age, reporting to the Department of Pediatric and preventive dentistry, Vinayaka Mission’s Sankarachariyar Dental College were collected based on the inclusion and exclusion criteria.
Inclusion criteria
The following criteria were included in the study:
Deciduous carious (deep dentinal caries) and noncarious teeth with more than two-thirds root resorption (based on findings by Sahara et al.[7])
Deciduous teeth with pre-shedding mobility, and
Over-retained deciduous teeth with and without dental caries.
The study was conducted over a period of 3 months, and the extracted teeth were preserved in 10% formalin till the start of the experiment.
Exclusion criteria
The following criteria were excluded from the study:
Healthy sound primary teeth with more than one-third of root remaining
Deciduous teeth with the absence of mobility and pain.
The samples were divided into two groups of 10 each: Group A – without dental caries (n = 10); Group B – with dental caries (n = 10).
Tissue preparation and staining
The obtained specimens were decalcified using 5% nitric acid for 48–72 h. Next, they were dehydrated with the aid of alcohol. Further, the samples were embedded in paraffin wax and sectioned. Staining was done with haematoxylin and eosin.
Analysis of the specimen
The stained and processed samples were observed under a light microscope with a magnification of ×400. Further assessment in regards to the shape and number of odontoclasts and pattern and nature of inflammatory cells in the concerned carious and non-carious specimens were done.
Statistical analysis
The intergroup comparison of the number of odontoclasts and inflammatory cells between Group A (without caries) and Group B (with dental caries) was done using the independent t-test. The level of significance was kept at P < 0.05.
RESULTS
The present study evaluated the number of odontoclastic cells and inflammatory cells in teeth with and without dental caries. Table 1 shows the number of odontoclasts and inflammatory cells in Group A (without dental caries). The odontoclasts were more commonly observed to have a polygonal, oval shape and the inflammatory cells were scanty. [Table 1 and Figure 1a].
| Specimen no. | No. of odontoclasts observed |
Shape of the odontoclasts | Nature of the inflammatory cells | Pattern of the inflammatory cells |
|---|---|---|---|---|
| 1 | 0 | Nil | Scanty* | NIL |
| 2 | 5 | Polygonal, Oval | Scanty | NIL |
| 3 | 6 | Polygonal, Oval | Scanty | NIL |
| 4 | 4 | Polygonal, Oval, Round | Very few** | NIL |
| 5 | 6 | Polygonal, Oval | Scanty | NIL |
| 6 | 4 | Polygonal, Oval | Scanty | NIL |
| 7 | 7 | Polygonal | Very few | NIL |
| 8 | 5 | Polygonal | Scanty | NIL |
| 9 | 6 | Polygonal, Oval | Scanty | NIL |
| 10 | 2 | Polygonal, Oval | Scanty | NIL |
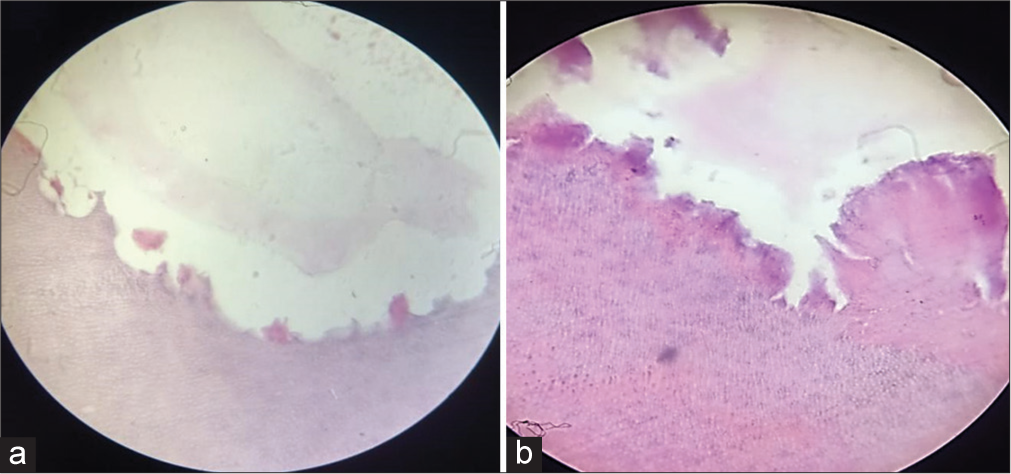
- (a) Group A specimen under ×400 magnification showing scanty inflammatory cells and odontoclastic activity. (b) – Group B specimen under ×400 magnification showing diffuse and patchy pattern of inflammatory cells and hyperemia.
Table 2 shows the properties of odontoclasts and inflammatory cells observed in Group B (with dental caries). The odontoclasts were more commonly observed to be polygonal in shape and very few inflammatory cells were observed [Table 2 and Figure 1b].
| Specimen no. | No. of odontoclasts observed |
Shape of the odontoclasts | Nature of the inflammatory cells | Pattern of the inflammatory cells |
|---|---|---|---|---|
| 1 | 2 | Polygonal, Oval | Very few** | Patchy |
| 2 | 3 | Polygonal | Very few | Patchy |
| 3 | 5 | Polygonal, Oval | Scanty* | Patchy |
| 4 | 3 | Polygonal | Very few | Patchy |
| 5 | 2 | Polygonal, Oval | Very few | Patchy |
| 6 | 2 | Polygonal | Very few | Patchy |
| 7 | 2 | Polygonal | Very few | Patchy |
| 8 | 2 | Polygonal | Very few | Patchy |
| 9 | 3 | Polygonal | Very few | Patchy |
| 10 | 3 | Polygonal, Oval | Very few | Patchy |
The number of odontoclasts observed in Group A (without dental caries) had a mean value of 4.5 ± 2.12 which was higher than in Group B (with dental caries), which showed a mean value of 2.70 ± 0.94. The mean value of inflammatory cells observed in Group A (without dental caries) was 1.20 ± 0.42, which was fewer than found in Group B (with dental caries) (2.10 ± 0.56) [Table 3 and Figure 2].
| Variable | Groups | Mean | Standard deviation | t-value | P-value |
|---|---|---|---|---|---|
| Odontoclasts observed | Group A - without dental caries | 4.50 | 2.12 | 2.44 | 0.02 |
| Group B - with dental caries | 2.70 | 0.94 | |||
| Inflammatory cells observed | Group A - without dental caries | 1.20 | 0.42 | 4.21 | 0.00 |
| Group B - with dental caries | 2.10 | 0.56 |
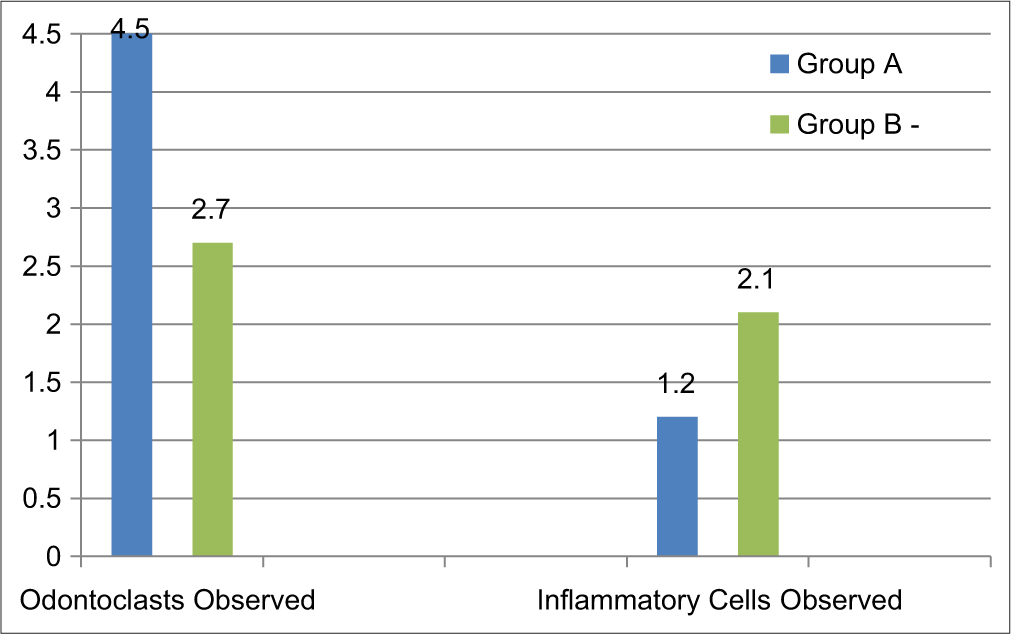
- Intergroup comparison of number of odontaclasts and inflammatory cells observed.
The intergroup comparison of odontoclasts observed showed a statistically significant difference between the two groups with p<0.05, and the difference of inflammatory cells observed was highly statistically significant between the groups (p value 0.00) [Table 3].
DISCUSSION
In the sample specimen, various stages of resorption were processed and observed under a light microscope.
Sahara et al.,[7] reported that odontoclasts are not found inside the pulp until root resorption is near completion. Thus, teeth with more than two-thirds resorption were considered in this study.
In the present study, the odontoclastic cells predominantly appeared polygonal and oval. The rounded appearance of cells was observed only in a single specimen without dental caries. In most samples, the lacunae showcased a thin haematoxyphilic zone. In zones where the odontoclasts were in contact with the tooth surface, the typical brush border appearance could be appreciated.
There was a statistically significant difference in the number of odontoclasts between the two groups with Group A (without caries) having a significantly higher number of cells. The specimens showed scanty inflammatory cells with higher odontoclastic activity [Figure 1a]. The findings of the present study are in accordance with the results of the previous histological studies done on deciduous teeth.[8-10]
The inflammatory cells in the given sample appeared with varying morphology, scattered across the field of observation.
There seemed to be no uniform pattern of distribution of these cells and a minimal number of cells were noticed in Group A (without caries) specimens. The Group B (with caries) specimens showcased a drastic change in the number of inflammatory cells and the pattern of occurrence was patchy. Hyperemia and necrosis are often witnessed during observation. A zone of internal resorption could be spotted occasionally [Figure 1b].
There was a highly significant difference in the number of inflammatory cells between the two groups with Group B (with caries) having a significantly higher number of cells. Bolan and Rocha[11] reported similar findings with necrosis, hyperemia, and internal resorption in teeth with dental caries. However, teeth without dental caries were reported to show more inflammatory cells than teeth with dental caries which is contradictory to the findings of the present study. Kronfeld,[12] Soskolne and Binstein,[13] and Eronat et al.,[14] also showed contradictory findings and correlated the findings to secondary infiltration occurring through gingival sulcus at the end stages of resorption.
Di Nicolo et al.,[15] concluded that the type of dentinal caries is a good indicator of the histological status of the pulp. Murthy et al.[16] showed irrespective of the nature of teeth in a particular stage of resorption, the ultrastructural pattern of all distinct samples of deciduous teeth presented similar features. On the contrary, the macroscopic pattern varied as the surface of involvement differs amongst the samples.
Limitations
Correlation between the changes in odontoclastic activity to the corresponding stage of resorption of the teeth or to the age and other clinical details of the patient are not included in the study
Limited sample size of the study.
CONCLUSION
The findings of the present study indicate the clear dominance of inflammatory cells in the carious specimen over the noncarious specimen. Odontoclast number and activity were found to be significantly predominant in non-carious teeth. Regarding the nature of odontoclasts, they appear as multi-nucleated cells embedded in the lacunae and polygonal in shape in both carious and non-carious deciduous teeth. These points of difference establish that dental caries in deciduous teeth influence normal physiologic resorption at the cellular level, thereby reducing the rate of resorption and delaying exfoliation.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Clinical Resources In: AAE Glossary of Endodontic Terms (9th ed). Illinois: American Association of Endodontists; 2019. p. :42.
- [Google Scholar]
- Pulpal status of human primary teeth with physiological root resorption. Int J Paediatr Dent. 2009;19:16-25.
- [CrossRef] [PubMed] [Google Scholar]
- The resorption processes of human deciduous teeth studied by light microscopy, microradiography and electron microscopy. Arch Oral Biol. 1968;13:417-31.
- [CrossRef] [PubMed] [Google Scholar]
- The role of dental pulp cells in resorption of deciduous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:113-20.
- [CrossRef] [PubMed] [Google Scholar]
- Root resorption--a multidisciplinary problem in dentistry In: Biological Mechanisms of Tooth Eruption and root Resorption. Massachusetts: EBSCO Media; 1988. p. :293-301.
- [Google Scholar]
- Cytodifferentiation of the odontoclast prior to the shedding of human deciduous teeth: An ultrastructural and cytochemical study. Anat Rec. 1996;244:33-49.
- [CrossRef] [Google Scholar]
- Odontoclastic resorption at the pulpal surface of coronal dentin prior to the shedding of human deciduous teeth. Arch Histol Cytol. 1992;55:273-85.
- [CrossRef] [PubMed] [Google Scholar]
- Kinetics of bone-resorbing activity in developing periapical lesions. J Dent Res. 1991;70:1362-6.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology of furcation lesions associated with pulp degeneration in primary molars. Pediatr Dent. 1987;9:279-82.
- [Google Scholar]
- Histopathologic study of physiological and pathological resorptions in human primary teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:680-5.
- [CrossRef] [PubMed] [Google Scholar]
- A histomorphological study of the shedding process of human deciduous teeth at various chronological stages. Arch Oral Biol. 1977;22:331-5.
- [CrossRef] [PubMed] [Google Scholar]
- Histological investigation of physiologically resorbing primary teeth using Ag-NOR staining method. Int J Paediatr Dent. 2002;12:207-14.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathology of the pulp of primary molars with active and arrested dentinal caries. J Clin Pediatr Dent. 2000;25:47-9.
- [Google Scholar]
- Ultrastructural morphologic pattern in the roots of deciduous teeth in different stages of physiologic resorption. Pediatr Dent J. 2020;30:215-23.
- [CrossRef] [Google Scholar]






