Translate this page into:
Controversies Surrounding Infective Endocarditis Prophylaxis Prior to Dental Procedures
*Author for correspondence
This article was originally published by Informatics Publishing and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The guiding principles for the prevention of Infective Endocarditis (IE) following dental procedures have been a debatable topic for a long time now. Evidently this has led to numerous amounts of research and consequently dental practitioners from across the world follow different prophylactic treatment regimens. At present, there are a few regimens that various health authorities have endorsed in their respective countries. There is a lack of substantial data in order to determine which regimen is better over the other, and unfortunately, that places the practitioner in a difficult situation to decide the best for his patient.
Keywords
Infective Endocarditis
Antibiotic prophylaxis
Dental procedures
Controversy
1. Introduction
IE is a rare infection with life threatening consequences. Many patients with congenital or acquired heart disease are at a higher risk of infection because of high pressure turbulent flow of blood in the heart causing endothelial damage (Lam, Jan, Sandor & Clokie, 2008; Colledge, Walker & Ralston, 2010). Despite the advances in diagnosis, chemotherapy, and surgical management of the infection, it is still associated with high morbidity and mortality rates. Since the release of the American Heart Association (AHA) guidelines for the prevention of IE in 1997, there has been a lot of debate and consequently research in order to determine the efficacy of antibiotic prophylaxis in patients at risk. Over a period of the past decade there have been a number of guidelines that have been put forth, however, the confusion still remains.
The purpose of this article is to describe the pathogenesis of IE and review the new guidelines on prophylaxis along with suggestions for practicing dentists.
2. Pathogenesis/Role of Dental Procedures
In 1909, Horder discovered the association between dental health and IE. He described it as ‘infection grafted upon a previously sclerosed endocardium..the source of the infecting agent, in most cases, is in the mouth’ (Durack, 1995). Any trauma to gingiva and its supporting structures during extractions, endodontic and other manipulative dental procedures was believed to release the microorganisms, particularly viridans group of streptococci into the bloodstream. The damaged endothelium undergoes a chemical reaction to produce cytokines, integrins and tissue factor, which in turn attracts platelets, fibronectin and monocytes (Widmer, Yok-Ai, Entenza & Moreillon, 2006). The transient bacteremia, causes the microflora to colonize the fibrin platelet complex (Figure 1), where they kindle further deposition of fibrin and platelets on their surface.
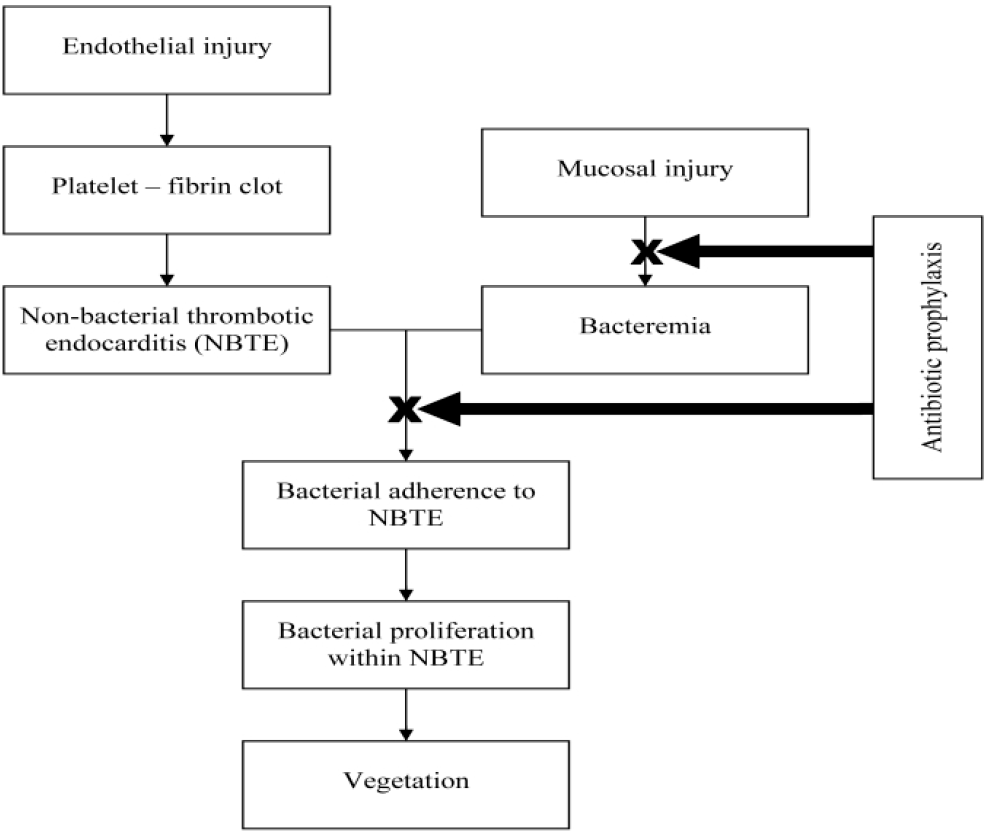
- Pathogenesis of IE.
The underlying microbes remain uninhibited by the host defense mechanism which eventually accelerates their multiplication. Thus, IE is induced as a result of complex interactions between the microbial pathogens in the bloodstream and the matrix molecules and platelets at sites of endocardial cell damage (Lam, Jan, Sandor & Clokie, 2008).
3. Organisms Associated with IE
Research has shown that although bacteremia was seen in 40% of patients after extractions, but only 3.6% of patients resulted with IE. 38% of patients also showed bacteremia after mastication and 11% of patients showed bacteremia with oral sepsis and no intervention (Guntheroth, 1984). Only a limited number of organisms have been discovered to cause post operative infections. These include, but are not limited to, Streptococcus viridans, Staphylococcus aureus, enterococcus, psueudomonas, serratia and candida (Wilson et al., 2007).
Highly virulent organisms (eg. Staphylococcus aureus) can also cause infection in a previously normal heart tissue by actively invading the endothelium causing apoptosis and endothelial damage. About 20% to 30% of individuals with community acquired staphylococcus bacteria develop IE (Yoav & Ethan, 2013).
4. Prophylaxis for IE during Dental Procedures
The age old saying of prevention is better than cure is followed religiously in the field of medical science. To prevent dental treatment from carving a way out for this deadly disease, a lot of the medical societies like AHA, British Society of Antimicrobial Chemotherapy (BSAC) and National Institute for Health and Clinical Excellence (NICE) recommended the use of antibiotic prophylaxis prior to dental procedures. AHA (1997) proposed the use of antibiotics prior to all dental procedures and to all patients at risk of IE, but the guidelines provided were empirical in approach and not based on clinical evidence (Wilson et al., 2007).
Over time, these guidelines were frequently challenged and numerous studies were performed to assess the validity of these guidelines and it was inferred that:
There was insufficient data in support of antibiotic prophylaxis being beneficial.
Antibiotic prophylaxis has its own risks and consequences. Penicillin causes allergic reactions among 1%–10% of patients. The incidence of death from anaphylactic reaction is seen to be approximately five times greater than that from treating IE (Lam, Jan and Sandor et al, 2008).
It was suggested that cumulative bacteremia caused by daily activities is more than that by a single dental procedure. Bacteremia from activities such as brushing, is estimated to be 6 million times higher than that occurring from a single tooth extraction (Ashrafian & Bogle, 2007). Preservation of optimal oral health and hygiene may decrease the prevalance of bacteremia from daily activities and is more important than prophylactic antibiotics for a dental procedure in reducing the risk of IE (Lam, Jan, Sandor & Clokie, 2008).
It has been observed that even if prophylaxis is 100% effective, only a minute number of cases of IE might be prevented by the antibiotic prophylaxis (Lam, Jan, Sandor & Clokie, 2008).
As a result, a revised set of guidelines (Table 1) for prophylaxis was proposed by AHA in 2007 and other societies as well, for use of antibiotics prior to dental procedures.
| Situation | Agent | Single Dose 30 to 60 min Before Procedure | |
|---|---|---|---|
| Adults | Children | ||
| Oral | Amoxicillin | 2 g | 50 mg/kg |
| Unable to take oral medication | Ampicillin OR | 2 g IM or IV | 50 mg/kg IM or IV |
| Cefazolin or Ceftriaxone | 1 g IM or IV | 50 mg/kg IM or IV | |
| Allergic to penicillins or ampicillin—oral | Cephalexin a,b OR | 2 g | 50 mg/kg |
| Clindamycin OR | 600 mg | 20 mg/kg | |
| Azithromycin or clarithromycin | 500 mg | 15 mg/kg | |
| Allergic to penicillin or ampicillin and unable to take oral medication |
Cefazolin or Ceftriaxoneb OR |
1 g IM or IV | 50 mg/kg IM or IV |
| Clindamycin | 600 mg IM or IV | 20 mg/kg IM or IV | |
Note: IM = intramuscular, IV = intravenous.
5. Guidelines by American Heart Association (AHA) in 2007
AHA proposed the use of prophylactic antibiotics only for high risk cardiac patients which included prosthetic cardiac valve, previous IE, congenital heart diseases (unrepaired cyanotic congenital heart disease, including palliative shunts and conduits), completely repaired congenital heart defect with prosthetic material or device, whether placed by surgery or by catheter intervention during the first 6 months after the procedure, repaired congenital heart disease with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization), Cardiac transplantation with subsequent cardiac valvulopathy (Lam, Jan, Sandor & Clokie, 2008).
AHA did not recommended antibiotic prophylaxis for any other cardiac conditions.
6. Guidelines by National Institute for Health and Clinical Excellence (NICE) in 2008
NICE is the only society to recommend no antibiotic prophylaxis for any cardiac patient undergoing a dental procedure. No coalition was found between the incidence of IE and the frequency of routine dental care within the previous year, tooth brushing or use of toothpicks (National Institute for Health and Clinical Excellence Clinical Guidelines 64 [NICE], 2008).
The NICE committee based their advice on the assertions that (Chambers et al., 2011).
There is no consistent and persistent association between having an interventional procedure, dental or non-dental, and the development of IE. Regular tooth brushing almost certainly caused more bacteremia and a greater risk of IE than a single dental procedure;
The clinical effectiveness and efficiency of antibiotic prophylaxis is not proven;
Antibiotic prophylaxis may cause higher number of fatalities due to anaphylactic reactions when used for dental procedures than a strategy of no antibiotic prophylaxis and is not cost effective.
7. Guidelines by Working Party of British Society of Antimicrobial Chemotherapy (BSAC) in 2006
Working Party of BSAC proposed another set of guidelines (Table 2) after reviewing the contemporary guidelines on endocarditis prophylaxis by American Heart Association, European Cardiac Society, and British Cardiac Society. This new set of guidelines was based on the evidence linking various procedures with the risk of bacterial endocarditis in susceptible individuals, changing spectrum of bacteria causing endocarditis and the section of individuals who acquire the disease despite been treated prophylactically.
| High risk cardiac factors requiring antibiotic prophylaxis | Dental procedures requiring antibiotic prophylaxis | Antibiotic regimens for endocarditis prophylaxis |
|---|---|---|
| Previous Infective Endocarditis Cardiac valve replacement surgery i.e. mechanical or biological prosthetic valves Surgically constructed systemic or pulmonary shunt or conduit |
All dental procedures involving dento-gingival manipulation. | Pre-operative mouth rinse with chlorhexidine gluconate 0.2% (10 ml for 1 minute) Adults and children ≥10 years Amoxicillin 3 g orally one hour before the dental procedure ≥ 5 < 10 years of age 1.5 g < 5 years of age 750 mg If Allergic to Penicillin Adults and children >10 years Clindamycin 600 mg orally one hour before the dental Procedure ≥ 5 < 10 years of age 300 mg < 5 years of age 150 mg Patients Allergic to Penicillin and Unable to Swallow Capsules Adults and children >10 years Azithromycin 500 mg orally one hour before the dental Procedure < 5 years of age 200 mg ≥ 5 < 10 years of age 300 mg Intravenous Regimens for Dental Treatment (When considered expedient) A single IV dose of 1 G amoxicillin ( <5 years of age 250 mg, ≥ 5 <10 years of age 500 mg) given just before the procedure or at induction of anaesthesia If Allergic to Penicillin A single IV dose of 300 mg clindamycin (given over at least 10 minutes) is recommended (<5 years of age 75 mg ≥ 5 <10 years of age 150 mg) Where a course of treatment involves several visits the antibiotic regimen should alternate between amoxicillin and clindamycin |
From “Guidelines for the prevention of endocarditis”, by F. K. Gould et al., 2006, Journal of Antimicrobial Chemotherapy, 57(6), p. 1035.
8. Discussion
All the three societies came forth with guidelines remarkably different from each other. To summaries the guidelines (Table 3).
| Guidelines | AHA, 1997 | ESC, 2004 | BSAC, 2005 | AHA, 2007 | NICE, 2008 |
|---|---|---|---|---|---|
| Risk groups based on cardiac conditions | High, Moderate, Negligible |
High, Moderate |
High | High | High |
| Risk group where prophylaxis is recommended/ optional |
High, Moderate |
High, Moderate |
High | High | – |
| Antiseptic rinse recommended |
Yes | Optional | Yes | No | No |
Notes: AHA-American Heart Association, ESC-European Society of Cardiology, BSAC-British Society of Anitimicrobial Chemotherapy, NICE- National Institute of Clinical Excellence. From “Infective endocarditis: rationale for revised guidelines for antibiotic prophylaxis.” By Gopalakrishnan, P. P., Sanjay, S. K., & Tak, T. (2009). Clinical Medicine & Research, 7, 63–68.
The new recommendations raise questions in multiple arenas. The AHA guidelines (2007), the NICE guidance (2008) and the British Society for Antimicrobial Chemotherapy (BSAC) guidelines (2005), represent compelling evidence of departure from the traditional approach to infective endocarditis prophylaxis (Gopalakrishnan, Sanjay & Tak, 2009). The areas of concern include withdrawal of the new guidelines from the traditional ones and also that the three aforementioned societies have come up with three considerably differentset of guidelines, even though each of them emphasize the lack of consistent evidence and proposes narrowing in antibiotic usage. AHA recommends prophylaxis for only dental and respiratory procedures and not gastrointestinal or genitourinary procedures, BSAC recommends for both dental and non dental procedures while NICE recommends prophylaxis for none of the procedures (Gopalakrishnan, Sanjay & Tak, 2009).
Theoretically, though the use of appropriate antibiotic prophylaxis for bacteremia inducing procedures in cardiac risk patients, should lead to a decreased incidence of IE, it has not been reflected in studies. As already noted in numerous amounts of published research, invasive dental procedures like extraction cause bacteremia, but the same has been proven to be true for routine daily procedures also. Studies have suggested that the vast majority of cases of IE infective endocarditis caused by oral micro flora are likely to be a result of random bacteremias caused by routine daily activities, such as flossing, tooth brushing, chewing food, use of water irrigation devices and other activities. These transient bacteremias usually clear within 10 minutes(Little, Falace, Miller & Rhodus, 2002). For example, brushing teeth was found to introduce bacteria into the bloodstream in about 40% of people tested, similarly, chewing paraffin or oral irrigation produced transient bacteremias in 50% of people (Little, Falace, Miller & Rhodus, 2002). Thus based on the high frequency of physiologic bacteremias and the low incidence of dental procedures preceding the onset of IE, the odds of a case of IE occurring from physiologic “seeding” of oral bacteria is 1,000 times greater than that after a dental procedure (Guntheroth, 1984). However, the presence of dental disease may increase the risk of bacteremia associated with these routine activities. Low-grade continuous bacteremia produced infections in experimental IE that were not significantly different from those resulting from transient high-grade bacteremia (Veloso et al., 2011). The cumulative exposure to bacteremia from routine daily activities in 1 year may be as high as 5.6 × 106 times greater as that resulting from a single tooth extraction (Roberts, 1999).
Studies have also demonstrated that only a small percentage of the IE cases were probably a result of dental procedures. Hence there is a lack of evidence in support of dental procedures being a high risk factor for development of IE and thus the necessity of antibiotic prophylaxis remains questionable.
Another set of controversies surround the risk of anaphylactic reactions associated with the use of Penicillin group of drugs. The British Society for Antimicrobial Chemotherapy report probably overestimates the risk of fatal anaphylaxis after an oral dose of amoxicillin. In contrast, the American Heart Association guidelines comment on the absence of any reports of fatal anaphylaxis associated with the antibiotic prophylaxis of endocarditis (Shanson, 2008). Also, the Cochrane Collaboration concluded that there is lack of evidence as to whether penicillin prophylaxis is in fact effective against IE in people at risk and who are about to undergo an invasive dental procedure. There is a lack of evidence again to support published guidelines in this area and it is not clear whether the potential harms and costs of penicillin administration outweigh any beneficial effect(Oliver, Roberts & Hooper, 2004); 1– 10% of the patients report a penicillin allergy and the chance of a penicillin reaction is about 5% for high doses of oral amoxicillin. It has been calculated that the in such a large unselected population of patients receiving prophylaxis, the risk of death from anaphylactic reactions is five times greater than from contracting IE (Ashrafian & Bogle, 2007).
There is also substantial data in support of the increasing antibiotic resistance developing due to imprudent use of antibiotics, especially by dentists. According to a report, dentists account for 7% of all antibiotic prescriptions in the world, with each dentist estimated to write an average of 4–5 prescriptions per week (Epstein, Chong & Le, 2000). Hence antibiotic stewardship by dentists is the need of the hour.
9. Conclusion
Since most of the studies have shown an inconsistent association between dental procedures and the development of IE, there is a need for clinical trials to testify the true efficacy of antibiotic prophylaxis prior to dental procedures. As health care providers, it is our moral responsibility to weigh the benefits before administering prophylactic antibiotics.
Risks associated with antibiotics include, but are not limited to, adverse drug reactions, the financial cost of antibiotics, development of super bug, and of course, medico-legal concerns. However, there is a general consensus that a conservative approach is advised to minimize the risk of developing resistance to current antibiotic regimens. Prophylactic use of antibiotics should be individual and evidence based and in consultation with a specialist.
Even though there are revised guidelines 2007 onwards, there lacks substantial scientific evidence to determine the true efficacy of administering antibiotics. While AHA and BSAC are still recommending protocols, NICE on the other hand decided to completely withdraw any regimen. Patients at risk should be informed about the benefits and risks of antibiotic prophylaxis along with emphasis on the significance of maintaining good oral health and educating the patients regarding the signs and symptoms of IE . Another interesting factor to consider here would be the practice of ‘defensive medicine’ that is on the increase these days. Do health professionals always prescribe medications that are necessary, or perhaps, at time, is the prescription made to avoid any medico-legal issues they may face otherwise?
Based on the revised guidelines, the number of patients receiving any form of prophylaxis has decreased significantly. However, patients who have received prophylaxis in the past and their dentists who support the prophylaxis regimen are definitely concerned about this change in path – especially in the UK, where NICE has done away with any prophylaxis. The regulating bodies in all countries also need to make sure that the current practitioners are in fact aware of the change in guidelines and fully understand the rationale behind the same in order to follow it.
We do not have enough data at this point in time to determine if these guidelines can be applied in developing countries such as India, where oral hygiene is relatively poor. All guidelines agree that the maintenance of excellent oral hygiene is one of the main measures for preventing IE. Therefore, more research needs to be done in India in order to determine the efficacy of the regimens in the set up here, and possibly in the future, developing a regimen that is appropriate for the at risk population. Evidence for chemoprophylaxis efficacy remains insufficient and necessitates further investigation. But the absence of evidence does not reflect evidence of absence and in such a balanced clinical situation, clinical discretion is of paramount importance.
10. Acknowledgement
We would like to thank Dr. Srikanth G, Assistant Professor from the Department of Oral and Maxillofacial Surgery for his guidance and support for this paper.
References
- (2007)Antimicrobial prophylaxis for endocarditis: emotion or Science. Heart. ;93:5-6.
- [CrossRef] [Google Scholar]
- (2011)Antibiotic prophylaxis of endocarditis: the rest of the world and NICE. Journal of the Royal Society of Medicine. ;104(4):138-140.
- [CrossRef] [Google Scholar]
- (2010). Davidson’s principles and practice of medicine Churchill Livingstone: Elsevier;
- [Google Scholar]
- (1995)Prevention of infective endocarditis. New England Journal of Medicine. ;332:38-44.
- [Google Scholar]
- (2000). A survey of antibiotic use in dentistry. Journal of American Dental Association. ;131(11):1600-1609.
- [Google Scholar]
- (1984)How important are dental procedures as a cause of infective endocarditis? American Journal of Cardiology. ;54(7):797-801.
- [Google Scholar]
- (2008)Prevention of infective endocarditis: revised guidelines from the American Heart Association and the implications for dentists. Journal of Canadian Dental Association. ;74(5):449-453.
- [Google Scholar]
- (2002). Infective endocarditis. In Dental management of the medically compromised patient. :21-51.
- [Google Scholar]
- National Institute for Health and Clinical Excellence Clini- cal Guidelines 64. 2008. Retrieved from www.nice.org.uk
- [Google Scholar]
- (2004). Penicillins for the prophylaxis of bacterial endocarditis in dentistry. The Cochrane database of systematic reviews [electronic resource]. 2:CD003813.
- [Google Scholar]
- (1999)Dentists are innocent! “Everyday” bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatric Cardiology. ;20(5):317-325.
- [Google Scholar]
- (2008). New British and American guidelines for the antibiotic prophylaxis of infective endocarditis: do the changes make sense? A critical review Current Opinion in Infectious Diseases. ;21(2):191-199.
- [CrossRef] [Google Scholar]
- (2011)Induction of experimental endocarditis by continuous low-grade bacteremia mimicking spontaneous bacteremia in humans. Infection and immunity. ;79(5):2006-2011.
- [Google Scholar]
- (2006)New concepts in the pathophysiology of infective endocarditis. Current Infectious Disease Reports. ;8(4):271-279.
- [Google Scholar]
- Quality of Care and Outcomes Research Interdisciplinary Working Group 2007 Prevention of infective endocarditis. Guidelines from the american health association, rheumatic fever, endocarditis, and kawasaki disease committee, council on cardiovascular disease in the young and the council on clinical cardiology, council on cardiovascular surgery and anesthesia and the quality of care and outcomes research interdisciplinary working group. Circulation. ;116(15):1736-1754.
- [Google Scholar]
- (2013)Pathophysiology of infective endocarditis. Current Infectious Disease Reports. ;15(4):342-346.
- [Google Scholar]






